Metallic Bonding (Cambridge (CIE) IGCSE Co-ordinated Sciences (Double Award)): Revision Note
Exam code: 0654 & 0973
Did this video help you?
Metallic bonding
The structure of a metal
Extended tier only
Metals consist of giant structures
Within the metal lattice, the atoms lose their outer electrons and become positively charged metal ions
The outer electrons no longer belong to any specific metal atom and are said to be delocalised
This means they can move freely between the positive metal ions and act like a “sea of electrons”
The metallic bond is the strong force of attraction between the positive metal ions and the delocalised electrons
This type of bonding occurs in metals and metal alloys, which are mixtures of metal
Diagram to show metallic bonding
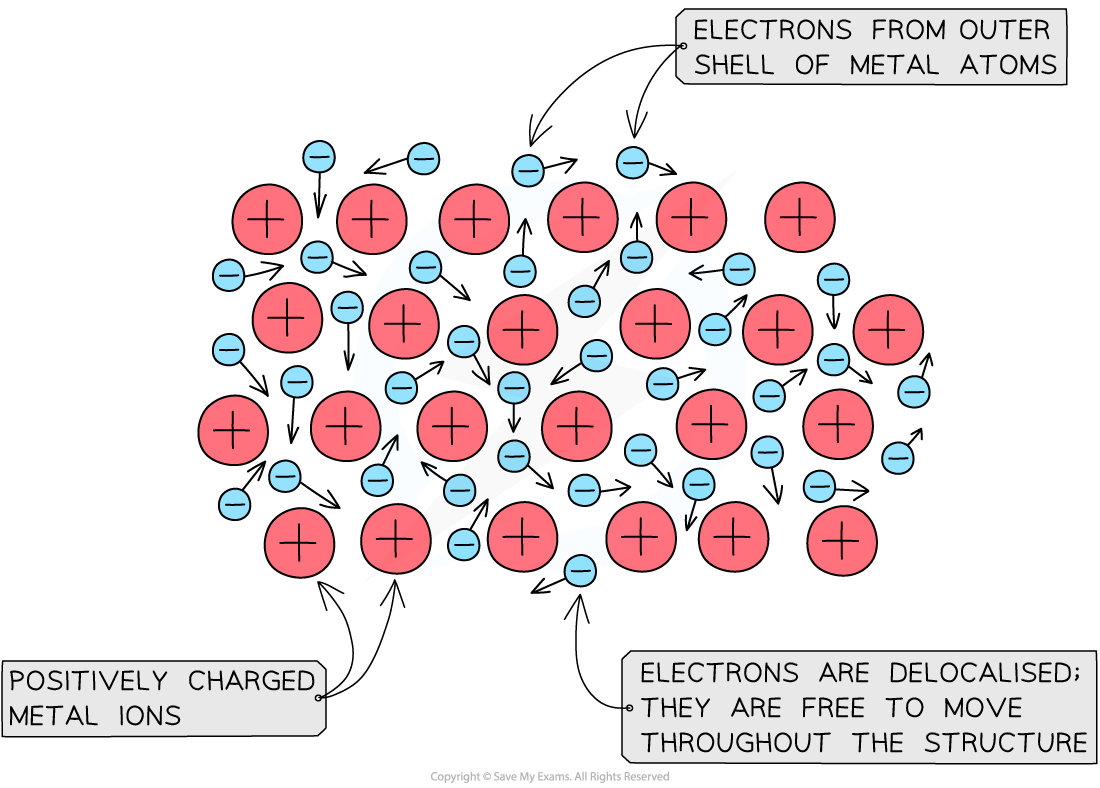
Diagram showing metallic lattice structure with delocalised electrons
Properties of metals
What are the properties of metals?
Extended tier only
Most metals have high melting and boiling points
There are strong electrostatic forces of attraction between the positive metal ions and the negative delocalised electrons within the metal lattice structure
These needs lots of energy to be broken
Metals are good conductors of heat and electricity
The delocalised electrons are free to move and carry a charge through the whole structure
Most metals are malleable
This means they can be hammered into shape
This is because the atoms are arranged in layers which can slide over each when force is applied
Malleability of metals

When a force is applied, the layers of positive ions slide over each other
Most metals are ductile
This means they can be pulled into wires
This is also because the atoms are arranged in layers which can slide over each when force is applied
Examiner Tips and Tricks
When explaining why metals can conduct electricity, be careful of the terminology you use. Don't get confused with ionic compounds.
Metals can conduct electricity as they have free electrons that can carry charge whereas molten or aqueous ionic compounds can conduct electricity because they have free ions that can carry charge.

Unlock more, it's free!
Did this page help you?