Collision Theory & Activation Energy (Oxford AQA IGCSE Chemistry) : Revision Note
Collision Theory
Particle theory states that chemical reactions occur only when the reactant particles collide with sufficient energy to react
The minimum amount of energy needed is called the activation energy
Activation energy is different for each reaction
Collisions can be described as successful or unsuccessful
A successful collision
A successful collision means that the reactant particles colliding have sufficient energy, i.e. greater than or equal to the activation energy
This means that the reactant particles rearrange to form the products
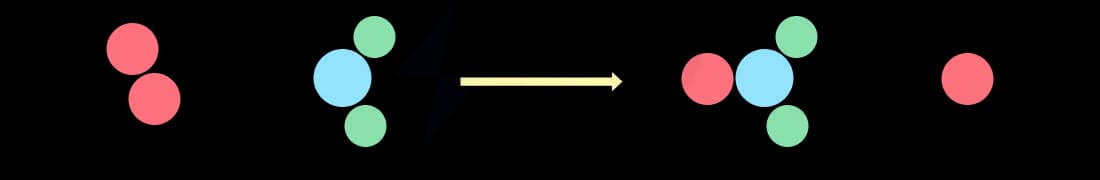
An unsuccessful collision
An unsuccessful collision means that the reactant particles have insufficient energy, i.e. less than the activation energy
This means that the reactant particles just bounce off each other and remain unchanged

Increasing the number of successful collisions means that a greater proportion of reactant particles collide to form product molecules
The following all affect the rate of reaction which is dependent on the number of successful collisions per unit time:
The number of particles per unit volume - more particles in a given volume will produce more frequent successful collisions
The frequency of collisions - a greater number of collisions per second will give a greater number of successful collisions per second
The kinetic energy of the particles - greater kinetic energy means a greater proportion of collisions will have an energy that exceeds the activation energy and the more frequent the collisions will be as the particles are moving quicker, therefore, more collisions will be successful
The activation energy - if a reaction has a high activation energy, there will be fewer collisions with an energy that exceeds the activation energy and fewer collisions will be successful
So, the rate of a reaction is dependent on the energy of collisions as well as the number of collisions

You've read 0 of your 5 free revision notes this week
Unlock more, it's free!
Did this page help you?

