Ionic Compounds (Oxford AQA IGCSE Chemistry) : Revision Note
Ionic Compounds
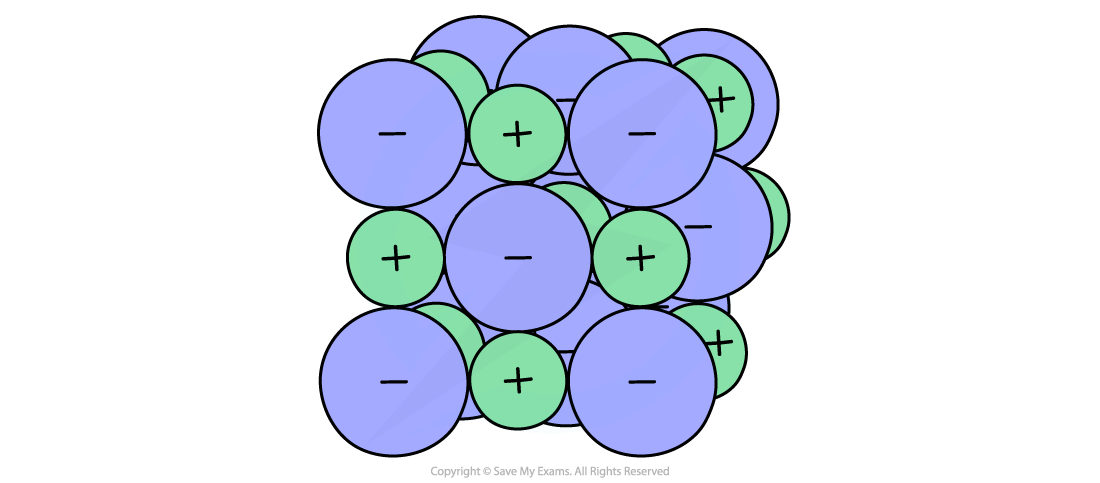
The lattices formed by ionic compounds consist of a regular arrangement of alternating positive and negative ions in which the ions are tightly packed together
Strong electrostatic forces of attraction are present between oppositely charged ions, holding the lattice together
Electrostatic forces are strong, acting in all directions - they form the basis of ionic bonding
The lattice structure of sodium chloride

The lattice arrangement exists in three dimensions which allows solid ionic compounds to form regular shapes
Solid ionic crystals contain huge numbers of ions and so are referred to as giant ionic lattices
Ions are incredibly small - a single grain of sodium chloride contains trillions of sodium and chloride ions - so models are used to represent the structure of the ionic compound
A 3D space-filling model showing an ionic lattice structure
Examiner Tips and Tricks
You need to be familiar with the structure of sodium chloride and be able to draw the structures of other ions given appropriate information.

You've read 0 of your 5 free revision notes this week
Unlock more, it's free!
Did this page help you?

