Substances can be classified as elements, compounds or mixtures.
Each of the boxes in the diagram represents either an element, a compound or a mixture.

i) Explain which two boxes represent an element.
(2)
ii) Explain which two boxes represent a mixture.
(2)
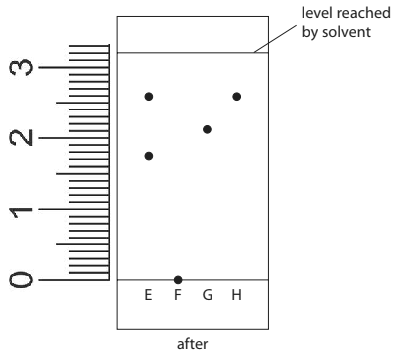
The list gives the names of some methods used in the separation of mixtures:
chromatography
crystallisation
distillation
filtration
Use names from the list to choose a suitable method for each separation. Each name may be used once, more than once or not at all.
i) Separating water from sodium chloride solution.
(1)
ii) Separating the blue dye from a mixture of blue and red dyes.
(1)
iii) Separating potassium nitrate from potassium nitrate solution.
(1)
Did this page help you?