Periodic Table: Basics (Edexcel IGCSE Chemistry (Modular)) : Revision Note
The periodic table
There are over 100 chemical elements which have been isolated and identified
Elements are arranged on the Periodic table in order of increasing atomic number
Each element has one proton more than the element preceding it
This is done so that elements end up in columns with other elements which have similar properties
The table is arranged in vertical columns called groups and in rows called periods
Period: These are the horizontal rows that show the number of shells of electrons an atom has and are numbered from 1 - 7
E.g. Elements in Period 2 have two electron shells, elements in Period 3 have three electron shells
Group: These are the vertical columns that show how many outer electrons each atom has and are numbered from 1 – 7, with a final group called Group 0 (instead of group 8)
E.g. Group 4 elements have atoms with 4 electrons in the outermost shell, Group 6 elements have atoms with 6 electrons in the outermost shell and so on
The periodic table
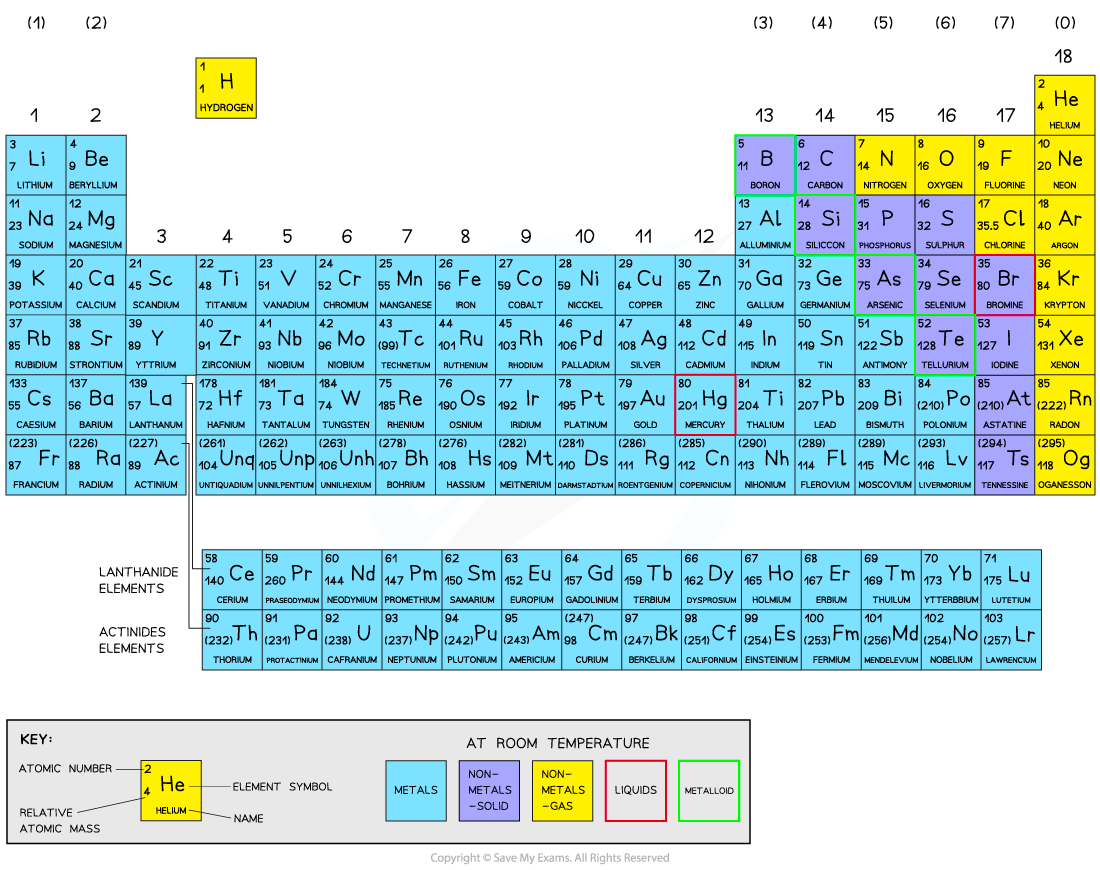
The Periodic Table of the Elements
Examiner Tips and Tricks
The atomic number is unique to each element and could be considered as an element's “fingerprint”.
The number of electrons changes during chemical reactions, but the atomic number does not change.

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?

