Galvanising & Sacrificial Protection (Cambridge (CIE) IGCSE Chemistry): Revision Note
Exam code: 0620 & 0971
Did this video help you?
Galvanising & sacrificial protection
Extended tier only
Sacrificial Protection
Iron can be prevented from rusting using the reactivity series
A more reactive metal can be attached to a less reactive metal
The more reactive metal will oxidise and therefore corrode first, protecting the less reactive metal from corrosion
Zinc bars on the side of steel ships
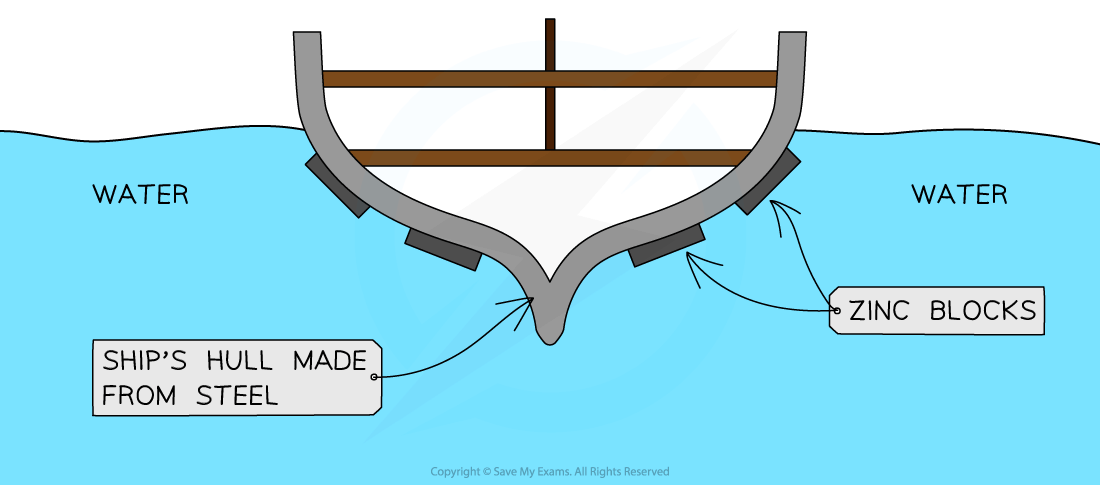
Diagram to show the use of zinc bars on the sides of steel ships as a method of sacrificial protection
Zinc is more reactive than iron therefore will lose its electrons more easily than iron and is oxidised more easily:
Zn → Zn2+ + 2e-
The iron is less reactive therefore will not lose its electrons as easily so it is not oxidised; the zinc is sacrificed to protect the steel
The zinc blocks do not need to cover the whole hull
As long as they are electrically connected to the steel, they can protect nearby areas
They are often placed on edges and joints where corrosion is most likely.
For continued protection, the zinc bars have to be replaced before they completely corrode
Galvanising
Galvanising is a process where the iron to be protected is coated with a layer of zinc
This can be done by electroplating or dipping it into molten zinc
ZnCO3 is formed when zinc reacts with oxygen and carbon dioxide in the air and protects the iron by the barrier method
If the coating is damaged or scratched, the iron is still protected from rusting by sacrificial protection
Examiner Tips and Tricks
You maybe asked to explain why a metal is/is not suitable as a method of preventing an iron/steel object from rusting. Remember that if it is higher in the reactivity series than iron, it will be suitable for sacrificial protection as it will be oxidised instead of iron. If it is lower in the reactivity series than iron, it would not be suitable as iron would be oxidised, causing it to rust.

Unlock more, it's free!
Was this revision note helpful?
