Properties of Metals (Cambridge (CIE) IGCSE Chemistry): Revision Note
Exam code: 0620 & 0971
Physical properties of metals & non-metals
The Periodic Table contains over 100 different elements
They can be divided into two broad types:
Metals
Non-metals
Most of the elements are metals and a small number of elements display properties of both types
These elements are called metalloids or semimetals
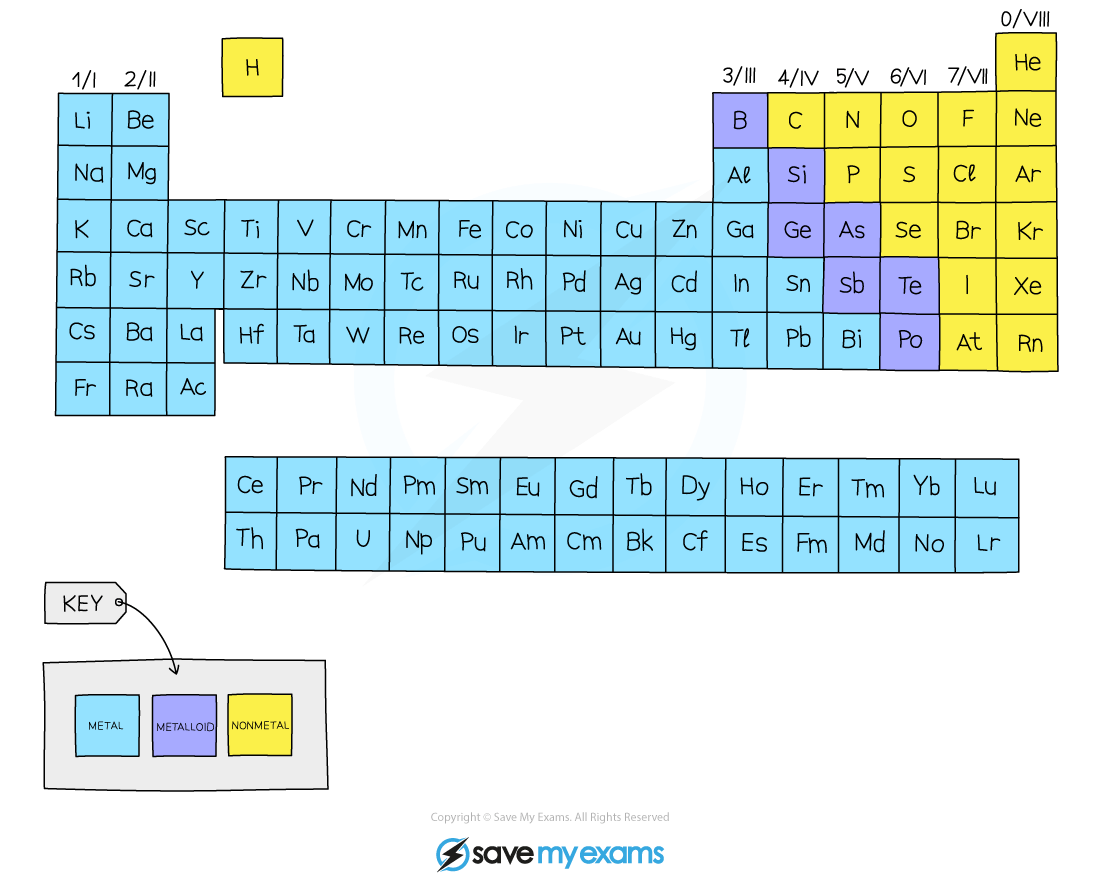
The metallic character diminishes moving left to right across the Periodic Table
Properties of metals
Conduct heat and electricity
This is because metals have delocalised electrons that are able to move through the metal structure
Are malleable (can be hammered and made into different shapes) and ductile (can be drawn into wires)
This is because the layers of positive metal ions, in the metal structure, are able to slide over each other
Usually have high melting and boiling points
This is because there is a strong electrostatic attraction between the positive metal ions and delocalised electrons (metallic bondmetallic bond)
This strong attraction / bond requires lots of energy to break
Properties of non-metals
Do not conduct heat and electricity
This is because all of the electrons are involved in covalent bonding
One exception to this is graphite
Are brittle when solid and easily break up
They are not malleable or ductile
One exception to this is graphite
Low melting and boiling points
Many non-metals are gases at room temperature
This is because they have weak forces between molecules
These weak intermolecular forces do not require a lot of energy to overcome
Exceptions to this include diamond and silicon(IV) dioxide
Chemical properties of metals
The chemistry of metals is studied by analysing their reactions with water, dilute acid and oxygen
Based on these reactions, a reactivity series of metals can be produced
Reactions of metals with water
Some metals react with water, either warm or cold, or with steam
Metals that react with cold water form a metal hydroxide and hydrogen gas
metal + water → metal hydroxide + hydrogen
For example, calcium:
Ca (s) + 2H2O (l) → Ca(OH)2 (aq) + H2 (g)
Metals that react with steam form a metal oxide and hydrogen gas
metal + water → metal oxide + hydrogen
For example, zinc:
Zn (s) + H2O (g) → ZnO (s) + H2 (g)
Reactions of metals with acids
Most metals react with acids, such as HCl
When acids and metals react, the hydrogen atom in the acid is replaced by the metal atom to produce a salt and hydrogen gas
metal + acid → salt + hydrogen
For example, iron:
Fe (s) + 2HCl (aq) → FeCl2 (aq) + H2 (g)
Reactions of metals with oxygen
Unreactive metals, such as gold and platinum, do not react with oxygen
Some reactive metals, such as the alkali metals, react easily with oxygen
Copper and iron can also react with oxygen, although much more slowly
When metals react with oxygen a metal oxide is formed
metal + oxygen → metal oxide
For example, copper:
2Cu (s) + O2 (g) → 2CuO (s)

Unlock more, it's free!
Did this page help you?