Preparing Soluble Salts (Cambridge (CIE) IGCSE Chemistry): Revision Note
Exam code: 0620 & 0971
Did this video help you?
Preparing soluble salts
How to name a salt
The name of salt has two parts:
The first part comes from the metal, metal oxide or metal carbonate used in the reaction
The second part comes from the acid
The name of the salt can be determined by looking at the reactants
For example, hydrochloric acid always produces salts that end in chloride and contain the chloride ion, Cl-
Other examples:
Sodium hydroxide reacts with hydrochloric acid to produce sodium chloride
Zinc oxide reacts with sulfuric acid to produce zinc sulfate
What is a salt?
A salt is a compound that is formed when the hydrogen atom in an acid is replaced by a metal or ammonium ion
For example, replacing H in HCl with potassium gives potassium chloride, KCl
Salts have many uses including:
Fertilisers
Batteries
Cleaning products
Healthcare products
Fungicides
The method used depends on:
The solubility of the salt being prepared
Whether the base is insoluble or soluble (alkali)
Preparing soluble salts
There are two main methods of preparing a soluble salt:
Method A: Using excess solid reactant
This includes three reactions:
Using excess metal
Using excess insoluble base
Using excess insoluble carbonate
Method B: Using an alkali (by titration)
This is for soluble bases such as sodium hydroxide
The method used depends on:
Whether the base is insoluble or soluble (alkali)
Whether a metal is used
Method A: Using excess solid reactant
This method is used when the reactant is a solid in excess. Any unreacted solid is removed by filtration, then the salt is crystallised.
Method A1: Using excess metal
This is for metals that react with dilute acids
Add dilute acid to a beaker
Add the metal in small pieces while stirring until no more reacts
This means that the metal is in excess
Filter the mixture to remove the excess metal
Transfer the filtrate (salt solution) to an evaporating basin
Heat the evaporating basin gently until the solution is concentrated
Check the solution is saturated by dipping a cold, glass rod into the solution and seeing if crystals form on the end
Leave the basin in a warm place to crystallize
If necessary, decant any excess liquid
Dry the crystals with filter paper
Example reaction - preparing magnesium sulfate crystals:
magnesium + sulfuric acid → magnesium sulfate + hydrogen
Mg (s) + H2SO4 (aq) → MgSO4 (aq) + H2 (g)
Method A2: Using excess insoluble base
This is for insoluble bases such as metal oxides
Warm dilute acid gently in a beaker
Add the insoluble base slowly while stirring until no more reacts
This means that the insoluble base is in excess
Filter the mixture to remove the excess insoluble base
Transfer the filtrate (salt solution) to an evaporating basin
Heat the evaporating basin gently until the solution is concentrated
Check the solution is saturated by dipping a cold, glass rod into the solution and seeing if crystals form on the end
Leave the basin in a warm place to crystallize
If necessary, decant any excess liquid
Dry the crystals with filter paper
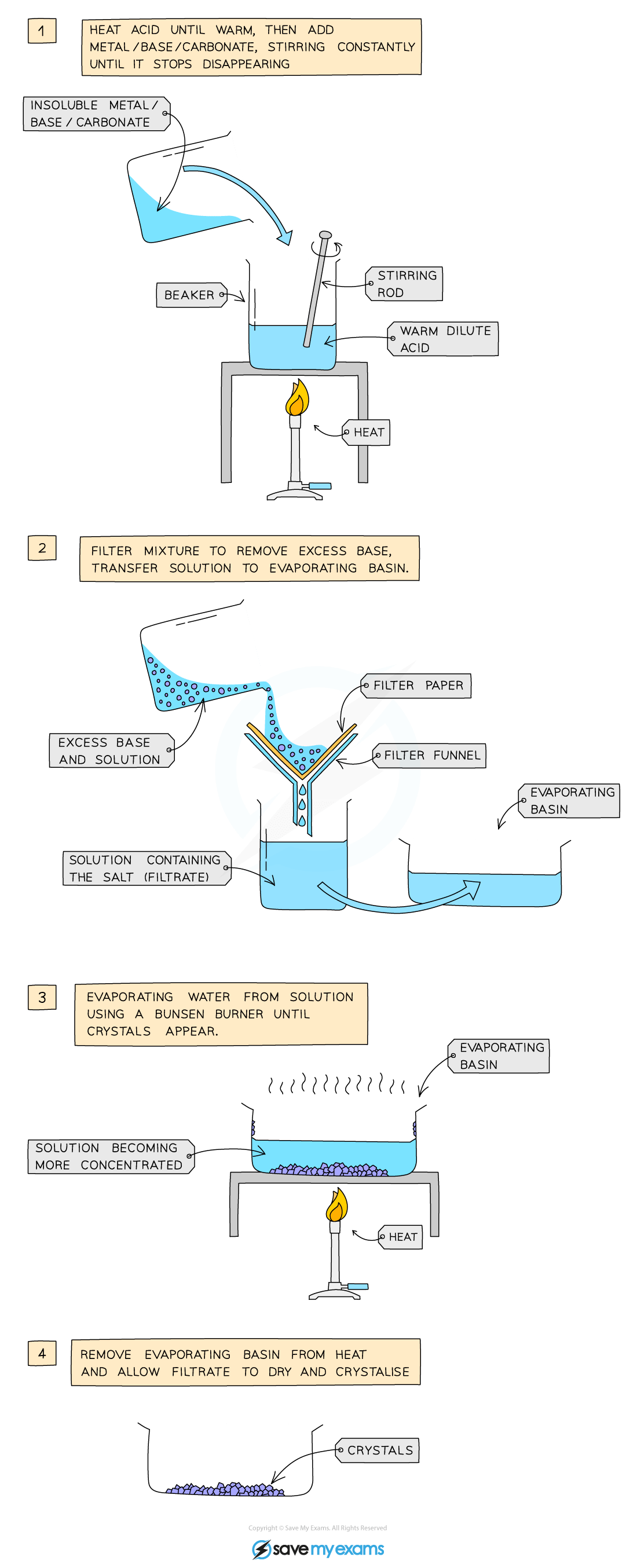
Example reaction - preparing copper(II) sulfate:
copper(II) oxide + sulfuric acid → copper(II) sulfate + water
CuO (s) + H2SO4 (aq) → CuSO4 (aq) + H2O (l)
Method A3: Using excess insoluble carbonate
This is for metal carbonates that do not dissolve in water
The method is exactly the same as using excess insoluble base:
Warm dilute acid gently in a beaker
Add the insoluble carbonate slowly while stirring until no more reacts (carbonate in excess)
Filter to remove the excess insoluble carbonate
Transfer the filtrate (salt solution) to an evaporating basin
Heat the evaporating basin gently until the solution is concentrated
Leave to crystallize, decant any excess liquid, and dry the crystals
Example reaction - preparing calcium chloride:
calcium carbonate + hydrochloric acid → calcium chloride + carbon dioxide + water
CaCO3 (s) + 2HCl (aq) → CaCl2 (aq) + CO2 (g) + H2O (l)
Method B: Using an alkali (by titration)
This is for soluble bases such as sodium hydroxide
Use a pipette to place alkali in a conical flask
Add a few drops of indicator (e.g. phenolphthalein)
Fill a burette with the acid and record the starting volume
Add the acid slowly while swirling until the indicator changes colour (end point)
Record the final volume
Repeat the titration without indicator, using the same measured volume of acid
Heat the neutral solution in an evaporating basin until concentrated
Check the solution is saturated by dipping a cold, glass rod into the solution and seeing if crystals form on the end
Leave the basin in a warm place to crystallize
If necessary, decant any excess liquid
Dry the crystals with filter paper
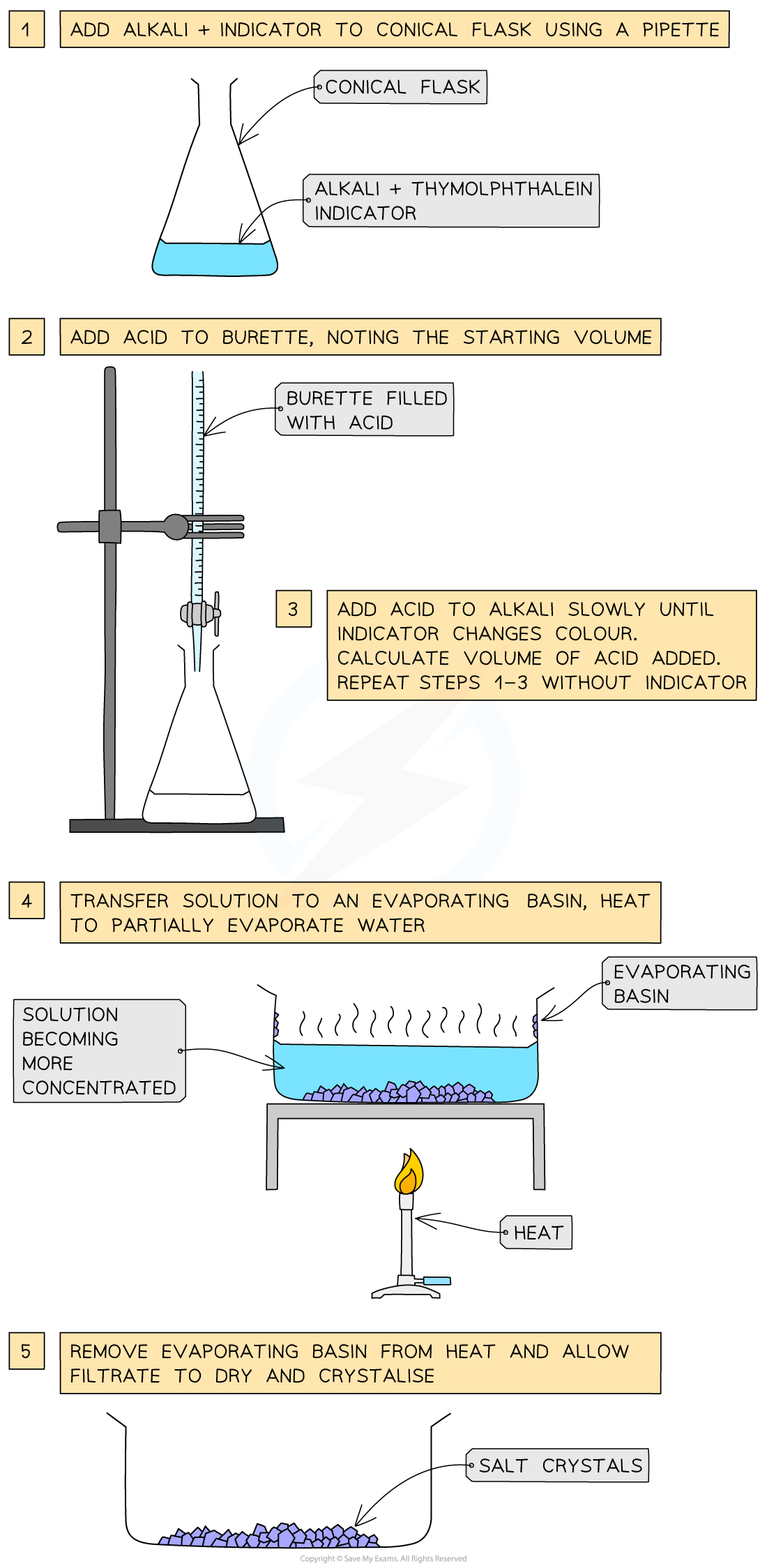
Example reaction - preparing sodium chloride crystals:
sodium hydroxide + hydrochloric acid → sodium chloride + water
NaOH (aq) + HCl (aq) → NaCl (aq) + H2O (l)
Examiner Tips and Tricks
Use Method B (titration) if the base is soluble (alkali)
Use Method A (excess solid) if adding a solid in excess, then filtering and crystallising:
A1 for excess metal
A2 for excess insoluble base
A3 for excess insoluble carbonate

Unlock more, it's free!
Was this revision note helpful?
