Reversible Reactions (Cambridge (CIE) IGCSE Chemistry): Revision Note
Exam code: 0620 & 0971
Did this video help you?
Reversible reactions
Some reactions go to completion, where the reactants are used up to form the product molecules and the reaction stops when all of the reactants are used up
In reversible reactions, the product molecules can themselves react with each other or decompose and form the reactant molecules again
It is said that the reaction can occur in both directions:
The forward reaction forming the products
The reverse reaction forming the reactants
If the forward reaction is exothermic, then the reverse reaction will be endothermic
The same amount of heat is transferred in both directions
Chemical equations for reversible reactions
When writing chemical equations for reversible reactions, the following symbol is used:
An example is, the reaction for the Haber process which produces ammonia from nitrogen and hydrogen
N2 + 3H2 ⇌ 2NH3
The forward reaction, producing ammonia, is exothermic
So, the reverse reaction is endothermic
Hydrated and anhydrous salts
Hydrated salts are salts that contain water of crystallisation which affects their molecular shape and colour
Water of crystallisation is the water that is stoichiometrically included in the structure of some salts during the crystallisation process
One example is copper(II) sulfate:
hydrated copper(II) sulfate ⇌ anhydrous copper(II) sulfate + water
CuSO4•5H2O ⇌ CuSO4 + 5H2O
The hydrated salt is copper(II) sulfate pentahydrate, CuSO4•5H2O
These are usually seen as blue crystals
The hydrated salt can be heated / dehydrated to form anhydrous copper(II) sulfate, CuSO4
This reaction is endothermic as energy is taken in to remove the water
The anhydrous salt is copper(II) sulfate
This is usually seen as white crystals / powder
Adding water to the anhydrous salt forms the hydrated copper(II) sulfate pentahydrate, CuSO4•5H2O
This reaction is highly exothermic
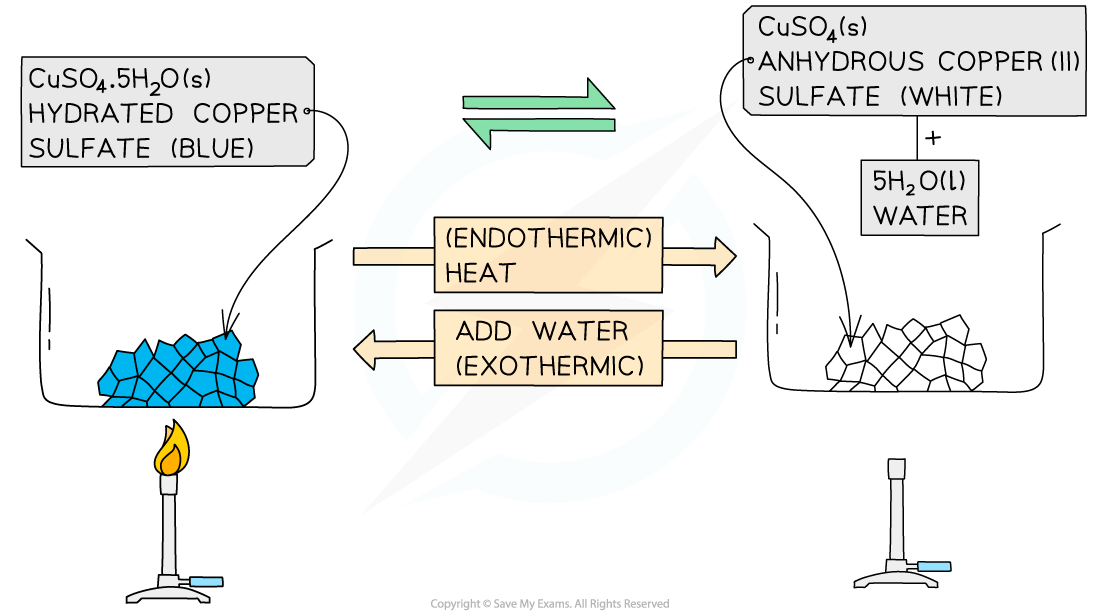
The forward reaction is exothermic and the reverse reaction is endothermic
Another example is cobalt(II) chloride:
hydrated cobalt(II) chloride ⇌ anhydrous cobalt(II) chloride + water
CoCl2•6H2O ⇌ CoCl2 + 6H2O
The hydrated salt is cobalt(II) chloride hexahydrate, CoCl2•6H2O
These are usually seen as pink crystals
The hydrated salt can be heated / dehydrated to form anhydrous cobalt(II) chloride, CoCl2
This reaction is endothermic as energy is taken in to remove the water
The anhydrous salt is cobalt(II) chloride, CoCl2
This is usually seen as blue crystals
Adding water to the anhydrous salt forms the hydrated cobalt(II) chloride hexahydrate, CoCl2•6H2O
This reaction is highly exothermic
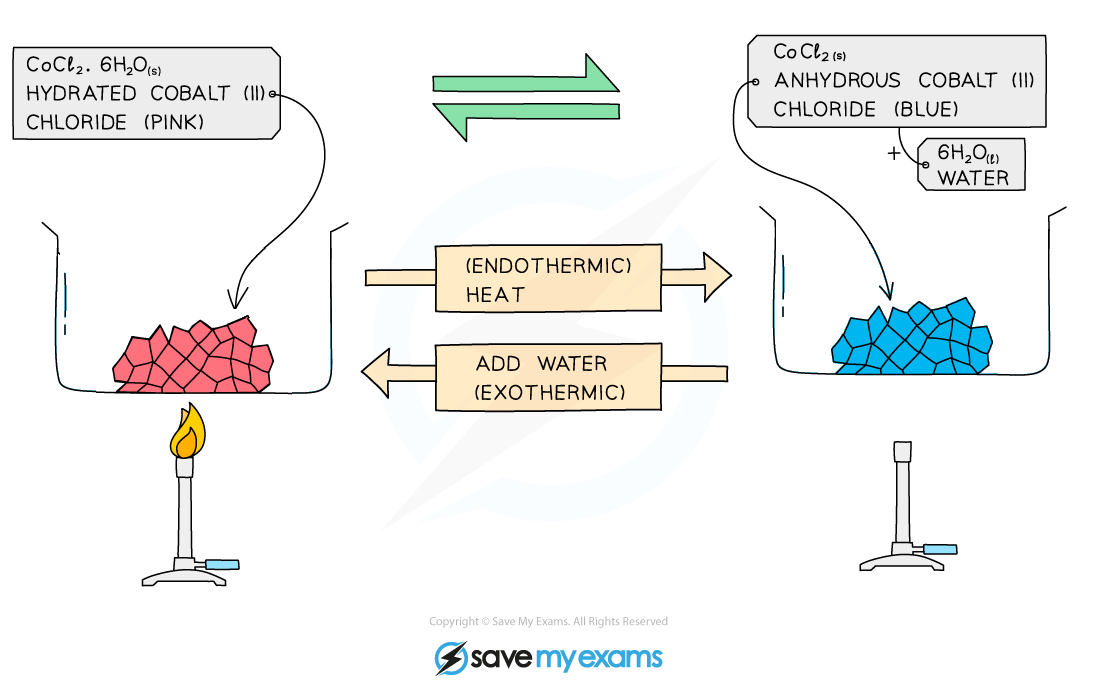
The forward reaction is exothermic and the reverse reaction is endothermic
Examiner Tips and Tricks
The hydration of CoCl2 and CuSO4 are chemical tests used to detect the presence of water.
You should remember the equations and colour changes:
CoCl2 + 6H2O ⇌ CoCl2.6H2O Blue to pink
CuSO4 + 5H2O ⇌ CuSO4.5H2O White to blue

Unlock more, it's free!
Did this page help you?