Interpreting Data (Cambridge (CIE) IGCSE Chemistry): Revision Note
Exam code: 0620 & 0971
Did this video help you?
Interpreting data
Data recorded in rate studies is used to plot graphs to calculate the rate of a reaction
Plotting a graph until the completion of the reaction shows how the rate of reaction changes with time
The rate of reaction is always quickest at the start of the chemical reaction
This is when there are the most reactants available to collide and form products
On a graph, the line at the start of the reaction has the steepest gradient
As the reaction progresses, the rate of reaction decreases
This is because the concentration of reactants decreases as they are being used up
On a graph, the line becomes less steep
The reaction eventually stops
This is because at least one of the reactants has been used up
The rate of reaction is now zero
On a graph, this is when the line becomes horizontal
The amount of product formed in a reaction is determined by the limiting reactant:
If the amount of limiting reactant increases, the amount of product formed increases
If the amount of the reactant in excess increases, the amount of product remains the same
You can plot more than one run of a variable on the same graph making it easier to see how the variable influences the rate
For example, plotting the effect of concentration on a reaction between the acid and marble chips

Drawing a tangent to the slope allows you to show the gradient at any point on the curve
A steeper slope means a quicker reaction and a higher rate of reaction
The volume of a gaseous product would increase to a maximum over time
So, the line levels out indicating the reaction is over
Since the volume and mass would be proportional, this could also be a graph of the mass of product versus time
Worked Example
0.2 g of manganese(IV) oxide was added to 25 cm3 of 0.1 mol / dm3 hydrogen peroxide solution. The volume of oxygen produced every minute was recorded and the results are shown on the graph.

The experiment was repeated using the same mass of manganese(IV) oxide and at the same temperature but using 25 cm3 of 0.2 mol / dm3 of hydrogen peroxide solution.
Sketch the curve for the results of this experiment on the same grid.
Answer:
Deduce how the initial gradient will be different from the original graph
The hydrogen peroxide solution is twice as concentrated
So, the rate of reaction will be greater and the initial gradient will be steeper
Deduce how much product will be formed compared to the original experiment
The amount of hydrogen peroxide determines the amount of oxygen produced
In the 2nd experiment, there are twice as many hydrogen peroxide molecules in the same volume
So, this will produce double the amount of oxygen in the same time
Sketch the graph

Calculating the rate of reaction at a particular point
To calculate the rate of reaction at any given time / point in a chemical reaction, you need to find the gradient of the curve at that time / point
To do this:
Draw a tangent to the curve
Calculate the change in the y-axis
Calculate the change in the x-axis
Calculate the gradient of the tangent using:
Rate of reaction (or gradient) =
Worked Example
Iodine and methanoic acid react in aqueous solution.
I2 (aq) + HCOOH (aq) → 2I− (aq) + 2H+ (aq) + CO2 (g)
The rate of reaction can be found by measuring the volume of carbon dioxide produced per unit time and plotting a graph as shown:
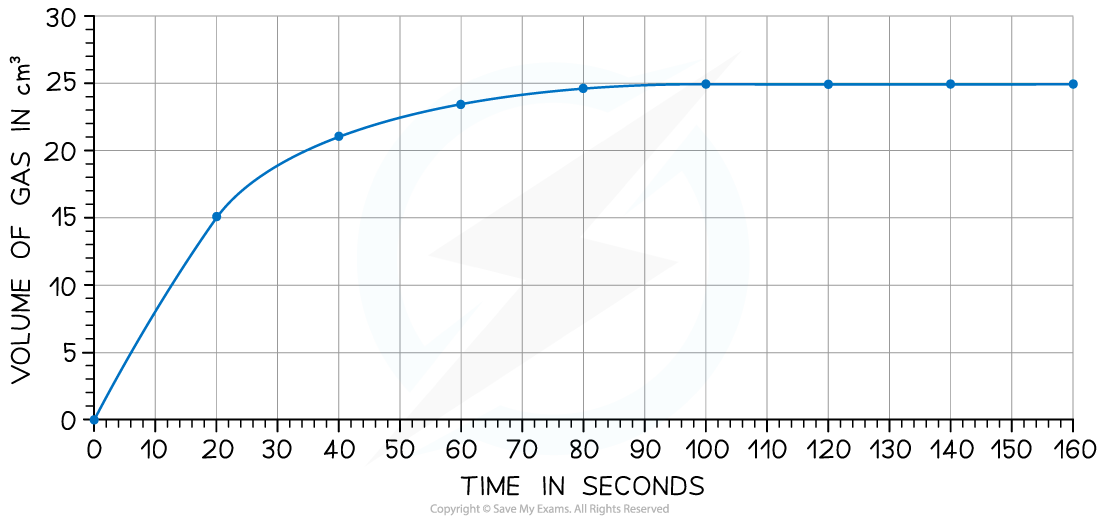
Calculate the rate of reaction at 20 seconds.
Answer:
Draw a tangent to the curve at 20 seconds:

Complete the triangle and read off the values of x and y
Determine the rate of reaction / gradient of the line:
Rate of reaction (or gradient) =
Rate of reaction (or gradient) =
= 0.60 cm3 / s
Examiner Tips and Tricks
If the amount of reactant used up is being monitored, then the graph will fall with the steepest gradient at the start, becoming less steep until it levels off to a horizontal line.

You've read 0 of your 5 free revision notes this week
Unlock more, it's free!
Did this page help you?