Electrolysis of Aqueous Solutions (Cambridge (CIE) IGCSE Chemistry): Revision Note
Exam code: 0620 & 0971
Did this video help you?
Electrolysis of aqueous solutions
Extended tier only
Aqueous solutions always have water, H2O, present
In the electrolysis of aqueous solutions, the water molecules dissociate producing H+ and OH– ions:
H2O ⇌ H+ + OH–
These ions are also involved in the process and their chemistry must be considered
We now have an electrolyte that contains ions from the compound plus ions from the water
Which ions get discharged and at which electrode depends on the relative reactivity of the elements involved
Concentrated and dilute solutions of the same compound give different products
For anions, the more concentrated ion will tend to get discharged over a more dilute ion
Positive electrode - anode
Negatively charged OH– ions and non-metal ions are attracted to the positive electrode
If halide ions (Cl–, Br–, I–) are present, the halogen is produced at the anode
The halide ions lose electrons and forms the halogen (chlorine, bromine or iodine)
If there are no halide ions but OH– ions are present, oxygen is produced at the anode
The hydroxide ions lose electrons and forms oxygen gas (and water)
In both cases, the other negative ion remains in solution
How concentration affects products at the anode
The concentration of the solution affects the ion being discharged:
If a concentrated halide solution is being electrolysed, the halogen forms at the anode
If a dilute halide solution is being electrolysed, oxygen forms at the anode
For example:
For concentrated barium chloride solution :
Cl– ions are discharged more readily than the OH– ions
So, chlorine gas is produced at the anode
For dilute barium chloride solution:
Only OH– ions are discharged
So, oxygen is produced at the anode
Negative electrode - cathode
Positively charged H+ and metal ions are attracted to the negative electrode but only one will gain electrons
Either hydrogen gas or metal will be produced
If the metal is above hydrogen in the reactivity series:
The ions of the more reactive metal remain in solution
This causes the less reactive hydrogen ions, H+, to be discharged
So, hydrogen will be produced and bubbling will be seen at the cathode
If the metal is below hydrogen in the reactivity series:
The less reactive metal ions are discharged
So, the metal is produced and this will be seen plating onto the cathode
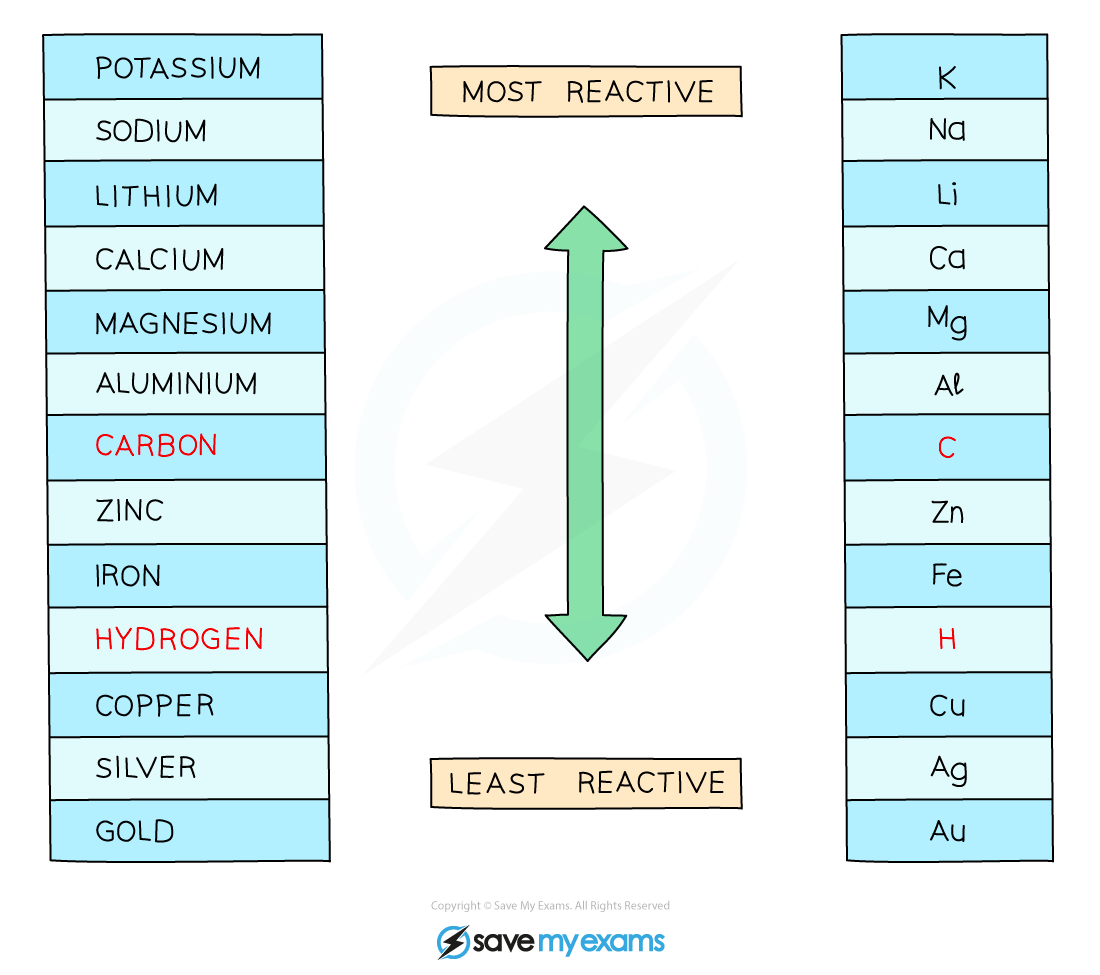
The reactivity series of metals including hydrogen and carbon
Electrolysis of aqueous copper sulfate
Aqueous copper sulfate contains the following ions:
Cu2+ and SO42– from the copper sulfate
H+ and OH– from the water
Using graphite electrodes:

Apparatus for the electrolysis of copper(II)sulfate using inert / passive graphite electrodes
Observations:
The cathode is coated with a pink-brown layer of copper metal
Bubbles of a colourless gas (oxygen) are seen forming at the anode
The blue colour of the copper(II) sulfate solution fades over time
Product at the cathode:
Cu2+ and H+ will both be attracted to the cathode but the less reactive ion will be discharged
In this case, copper is less reactive than hydrogen
Copper ions are discharged at the cathode
They gain electrons and are reduced to form copper metal
The half equation for the reaction at the electrode is:
Cu2+ + 2e– → Cu
Product at the anode:
SO42– and OH– are both attracted to the anode
OH– ions lose electrons more readily than SO42-
OH– lose electrons and are oxidised to form oxygen gas
The half equation for the reaction at the anode is
4OH– ⟶ O2 + 2H2O + 4e–
Using copper electrodes:
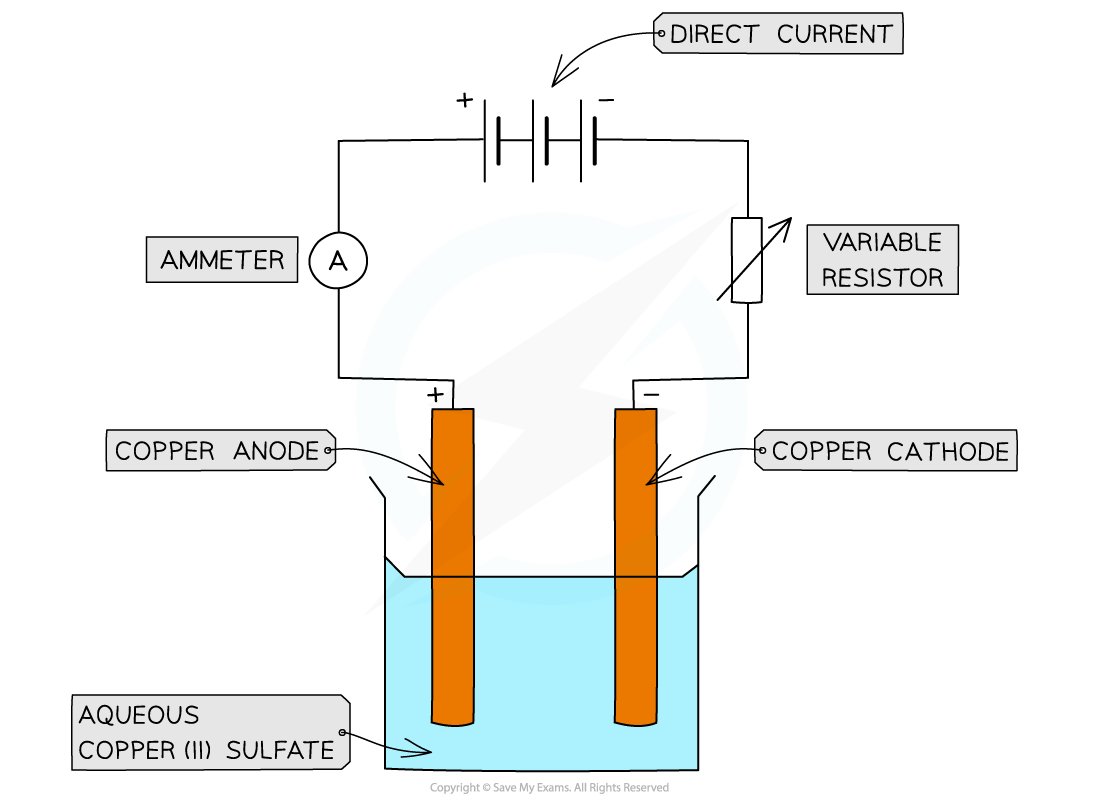
Apparatus for the electrolysis of copper(II)sulfate using active copper electrodes
Observations at the anode and cathode:
The blue colour of the copper(II) sulfate solution remains unchanged
The cathode increases in mass as it is coated with a layer of copper
This is because copper ions, Cu2+, are reduced at the cathode and form copper atoms
The anode decreases in mass as it dissolves
This is because copper atoms are oxidised at the anode and form copper ions, Cu2+
The gain in mass by the negative electrode is the same as the loss in mass by the positive electrode
Therefore, the copper deposited on the negative electrode must be the same copper ions that are lost from the positive electrode
This implies that the concentration of the Cu2+ ions in the solution remains constant
Products formed for common aqueous solutions
Aqueous solution - ions present | Product at the anode | Product at the cathode |
|---|---|---|
Concentrated sodium chloride, NaCl | Chlorine gas | Hydrogen gas |
Dilute sodium chloride, NaCl | Oxygen gas | Hydrogen gas |
Concentrated aqueous copper(II) sulfate, CuSO4 | Oxygen gas | Copper |
Dilute sulfuric acid, H2SO4 | Oxygen gas | Hydrogen gas |

Unlock more, it's free!
Did this page help you?