Empirical Formulae & Formulae of Ionic Compounds (Cambridge (CIE) IGCSE Chemistry): Revision Note
Exam code: 0620 & 0971
Empirical formulae
Extended tier only
The empirical formula is the simplest whole number ratio of the atoms of each element present in one molecule or formula unit of the compound
The empirical formula of an organic molecule is often different to its molecular / chemical formula
For example, ethanoic acid has the chemical formula CH3COOH or C2H4O2 but its empirical formula is CH2O
The molecular / chemical formula of an ionic compound is always its empirical formula
For example, sodium chloride has the chemical formula NaCl, which is also its empirical formula
Worked Example
Complete the table to give the molecular and empirical formulae of the given compounds.
Chemical | Molecular formula | Empirical formula |
 |
|
|
 |
|
|
 |
|
|
Answers:
The completed table is:
Chemical | Molecular formula | Empirical formula |
 | C3H8 | C3H8 |
 | C2H4 | CH2 |
 | C4H10O | C4H10O |
The first compound contains 3 carbon atoms and 8 hydrogen atoms
This 3:8 ratio of atoms cannot be simplified
Therefore, the molecular and empirical formula are both C3H8
The second compound contains 2 carbon atoms and 4 hydrogen atoms
This 2:4 ratio of atoms can be simplified to 1:2
Therefore, the molecular formula is C2H4 and the empirical formula is CH2
The third compound contains 4 carbon atoms, 10 hydrogen atoms and 1 oxygen atom
This 10:4:1 ratio of atoms cannot be simplified
Therefore, the molecular and empirical formula are both C4H10O
Did this video help you?
Deducing formulae of ionic compounds
Extended tier only
Metals and non-metals react together to form ionic compounds
Ionic compounds are not simple molecules
Remember: Simple molecules are formed when non-metals react together to form compounds
Ionic compounds involve the metal losing electrons and the non-metal gaining electrons to form ions
Some ions that you will be expected to be able to use, because they are stated in the exam specification, include:
Hydrogen ions, H+ - sometimes referred to as protons
Group 1 ions, e.g. Li+, Na+, K+
Group 7 ions, F–, Cl–, Br–
Copper(II) ions, Cu2+
Iron(II) ions, Fe2+
Iron(III) ions, Fe3+
There are some polyatomic (containing more than one atom) ions stated in the exam specification:
Carbonate ions, CO32–
Sulfate ions, SO42–
Hydroxide ions, OH–
Nitrate ions, NO3–
Ammonium ions, NH4+
How to determine the formulae of ionic compounds
Ionic compounds typically have no overall charge
This means that the size of any positively charged ion is cancelled by the size of any negatively charged ion
Careful: This should not be confused with an atom having no overall charge
Direct comparison
The formula of an ionic compound can be determined by directly comparing the charges of the ions:
For example, iron(II) sulfate
The iron(II) ion is Fe2+, which means that it has a 2+ or +2 charge
The sulfate ion is SO42–, which means that it has a 2– or –2 charge
The charges cancel each other out
Mathematically, (+2) + (–2) = 0
This means that one SO42– ion is needed to cancel the +2 charge on Fe2+
Therefore, the formula of iron(II) sulfate is FeSO4
The swap-and-drop method
When the ions in the ionic compound have different charges, it can be easier to use the swap-and-drop method
Careful: If you use this method with ions that have the same charge, then you must give the simplest whole number ratio to get the correct answer
For example, copper(II) chloride:
The copper(II) ion is Cu2+, which means that it has a 2+ or +2 charge
The chloride ion is Cl–, which means that it has a 1– or –1 charge
The size of the charge on the copper(II) ion indicates the number of chloride ions needed, and the size of the charge on the chloride ion indicates the number of copper(II) ions needed
Determining the formula of copper(II) chloride

The charges swap from element to element and drop down. The positive and negative signs are removed and there is no need for the number 1.
This gives the overall formula of copper(II) chloride as CuCl2
Worked Example
The compound produced in the reaction between iron wool and chlorine contains the ions Fe3+ and Cl–.
a) Give the formula of this compound.
b) State the name of this compound.
Answers:
Part a)
Direct comparison method:
The iron ion is Fe3+, which means that it has a 3+ or +3 charge
The chloride ion is Cl–, which means that it has a 1– or –1 charge
The charges do not cancel each other out
Mathematically, (+3) + (–1) ≠ 0
Three Cl– ions are needed to cancel the +3 charge on Fe3+
Therefore, the formula is FeCl3
Swap-and-drop method
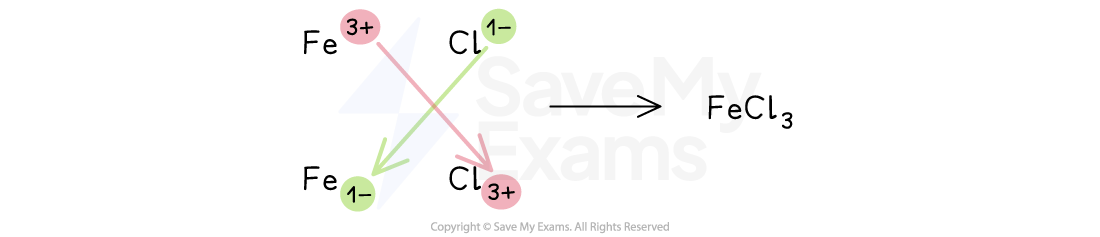
The formula is FeCl3
Part b)
The metal is iron and the chlorine will change to chloride
Therefore the name is iron chloride
Examiner Tips and Tricks
Take your time determining the chemical formula of ionic compounds with
Different charges on the ions
Polyatomic ions

Unlock more, it's free!
Did this page help you?