Molecules & Compounds (Cambridge (CIE) IGCSE Chemistry): Revision Note
Exam code: 0620 & 0971
Did this video help you?
Covalent bonds in complex covalent molecules
Extended tier only
Some atoms need to share more than one pair of electrons to gain a full outer shell of electrons
If two adjacent atoms share two pairs of electrons, two covalent bonds are formed, also known as a double bond
If two adjacent atoms share three pairs of electrons, three covalent bonds are formed, also known as a triple bond
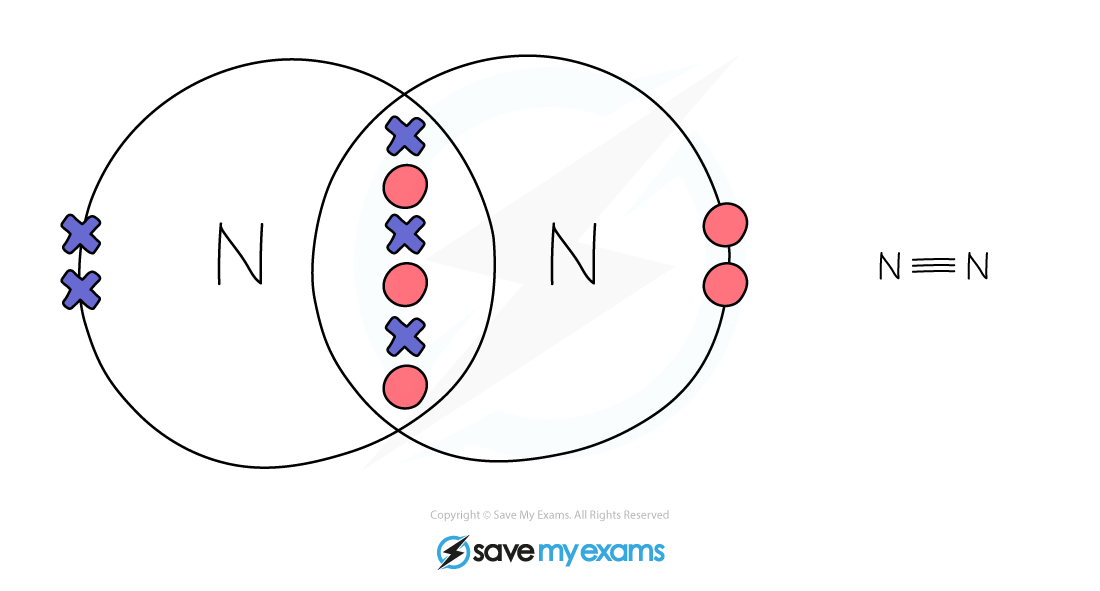
Nitrogen:
When 2 nitrogen atoms react they share 3 pairs of electrons to form a triple bond
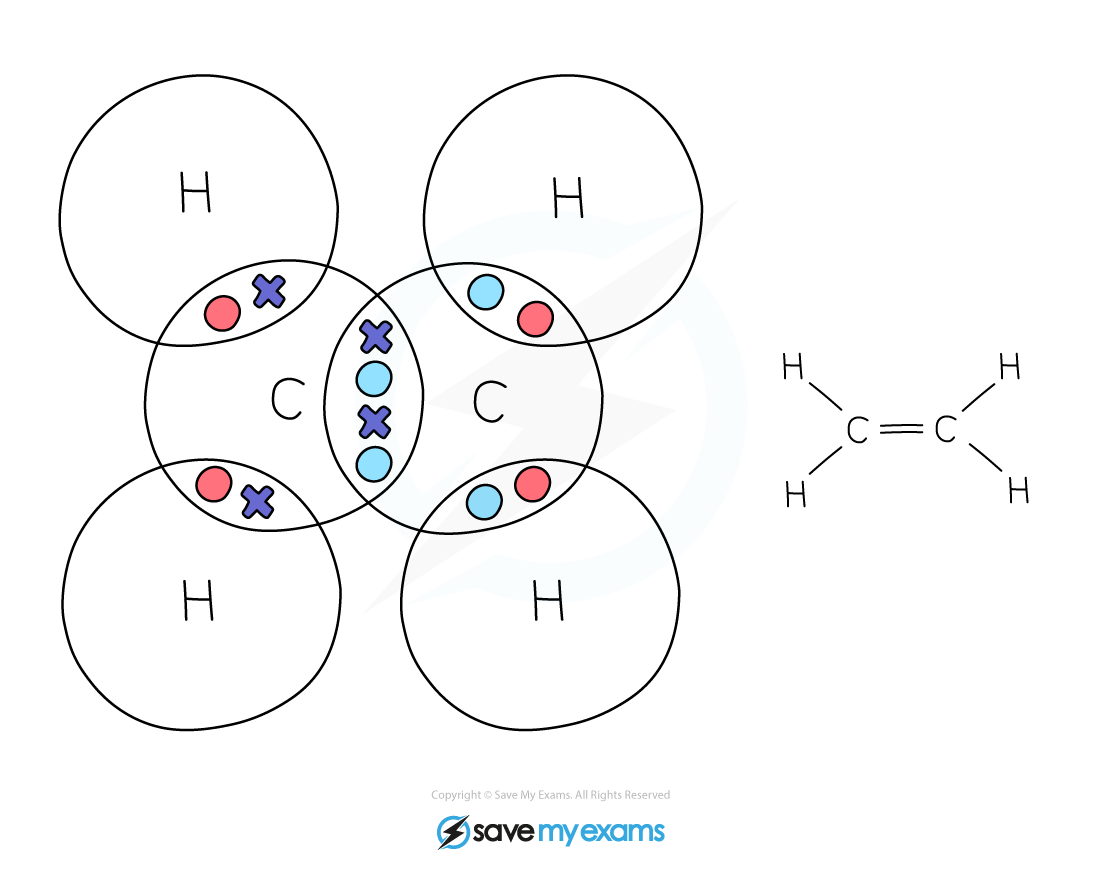
Ethene:
In ethene, the 2 carbon atoms share 2 pairs of electrons
This is known as a double bond
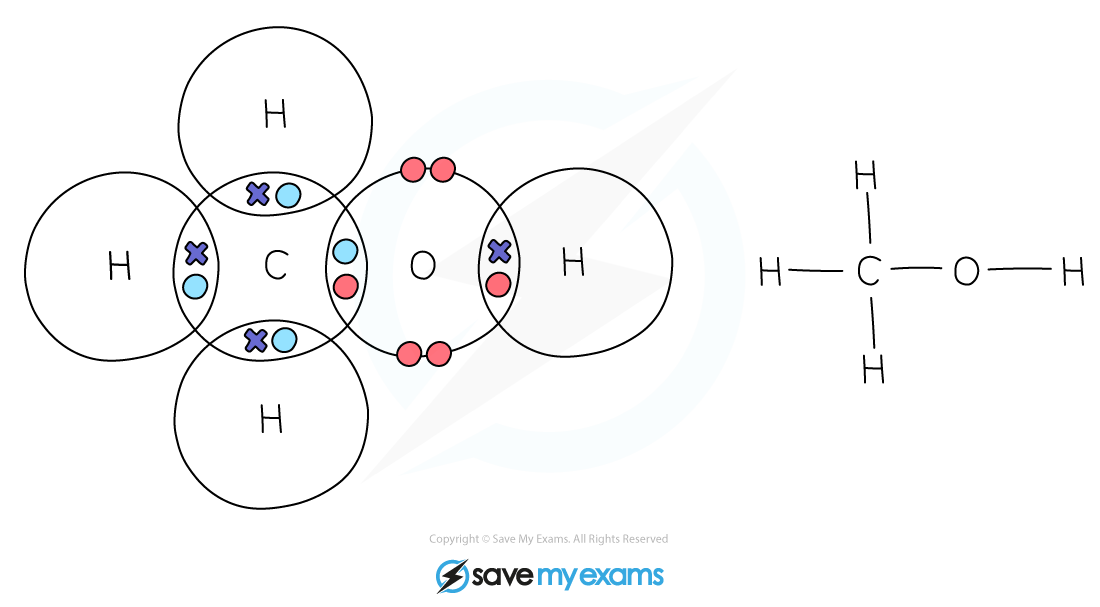
Methanol:
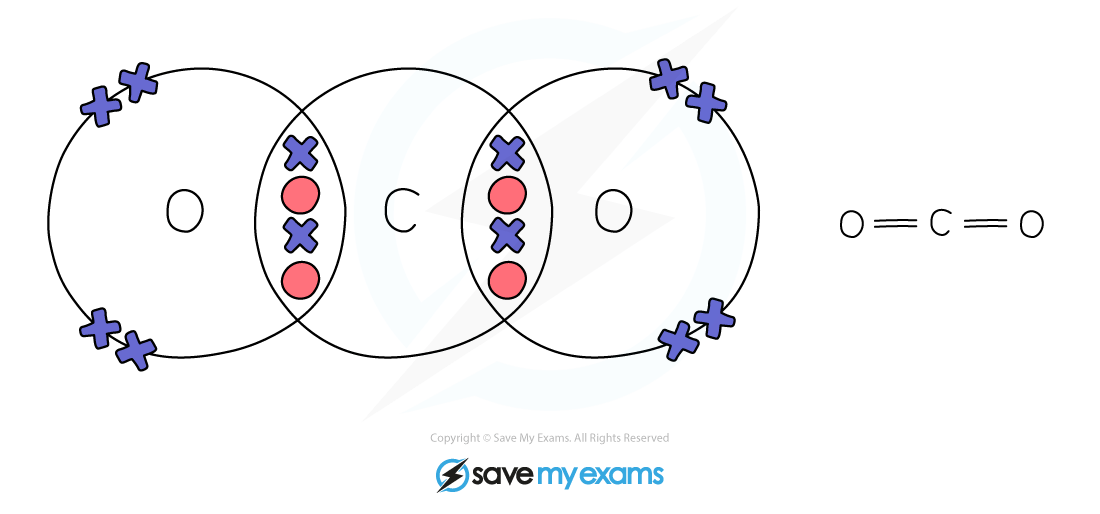
Carbon Dioxide:
Examiner Tips and Tricks
Be careful when drawing dot-and-cross diagrams, it is a common mistake for students to draw the wrong type of diagram.
Remember, if the compound contains metal and non-metal, it is an ionic compound and you need to draw the ions separated, with square brackets around each ion, together with a charge.
If the compound contains non-metal atoms only, it is a covalent compound, the shells should overlap and contain one or more pairs of electrons.

Unlock more, it's free!
Did this page help you?