Atomic Structure (Cambridge (CIE) IGCSE Chemistry): Revision Note
Exam code: 0620 & 0971
Did this video help you?
What is atomic structure?
All substances are made of tiny particles of matter called atoms which are the building blocks of all matter
Each atom is made of subatomic particles called protons, neutrons, and electrons
The protons and neutrons are located at the centre of the atom, which is called the nucleus
The electrons move very fast around the nucleus in orbital paths called shells
The mass of the electron is negligible, hence the mass of an atom is contained within the nucleus where the protons and neutrons are located
The structure of an atom

The structure of the carbon atom
Did this video help you?
Protons, neutrons & electrons
The size of atoms is so tiny that we can't really compare their masses in conventional units such as kilograms or grams, so a unit called the relative atomic mass is used
One relative atomic mass unit is equal to 1/12th the mass of a carbon-12 atom.
All other elements are measured relative to the mass of a carbon-12 atom, so relative atomic mass has no units
Hydrogen for example has a relative atomic mass of 1, meaning that 12 atoms of hydrogen would have exactly the same mass as 1 atom of carbon
Table of subatomic particles
Particle | Relative mass | Charge |
|---|---|---|
Proton | 1 | 1+ |
Neutron | 1 | 0 (neutral) |
Electron | 1- |
Examiner Tips and Tricks
Knowing the exact mass of an electron is not in the specification and saying it is almost nothing or negligible will be sufficient. It does, however, sometimes appear in particle identification questions, but you can usually deduce that it is the electrons from other information in the question.
Defining proton number
The atomic number (or proton number) is the number of protons in the nucleus of an atom
The symbol for atomic number is Z
It is also the number of electrons present in a neutral atom and determines the position of the element on the Periodic Table
Defining mass number
The nucleon number (or mass number) is the total number of protons and neutrons in the nucleus of an atom
The symbol for nucleon number is A
The nucleon number minus the proton number gives you the number of neutrons of an atom
Note that protons and neutrons can collectively be called nucleons.
The atomic number and mass number of an element can be shown using atomic notation
The Periodic Table shows the elements together with their atomic (proton) number at the top and relative atomic mass at the bottom - there is a difference between relative atomic mass and mass number, but for your exam, you can use the relative atomic mass as the mass number (with the exception of chlorine)
Atomic number & mass number diagram
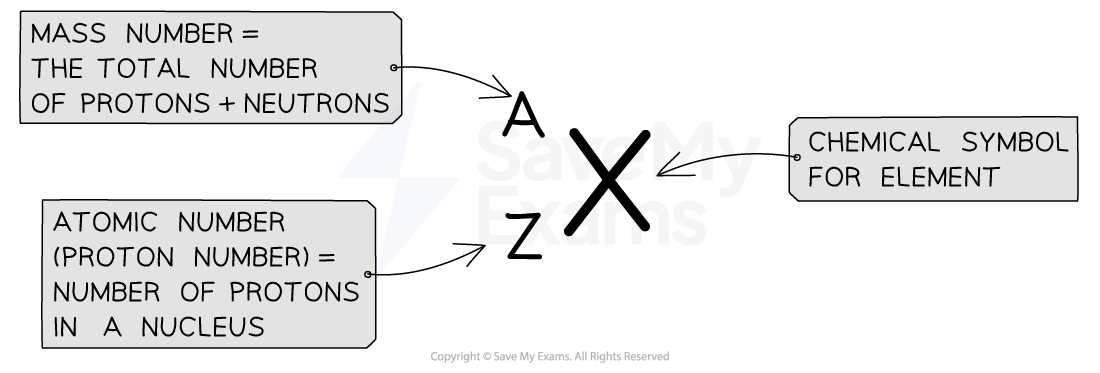
Diagram showing the notation used on the periodic table
How to calculate numbers of protons, neutrons and electrons
Protons
The atomic number of an atom and ion determines which element it is
E.g. lithium has an atomic number of 3 (three protons) whereas beryllium has atomic number of 4 (four protons)Therefore, all atoms and ions of the same element have the same number of protons (atomic number) in the nucleus
The number of protons equals the atomic (proton) number
The number of protons of an unknown element can be calculated by using its mass number and number of neutrons:
Mass number = number of protons + number of neutrons
Number of protons = mass number – number of neutrons
Electrons
An atom is neutral and therefore has the same number of protons and electrons
Neutrons
The mass and atomic numbers can be used to find the number of neutrons in ions and atoms:
Number of neutrons = mass number – number of protons
Worked Example
Determine the number of protons, electrons and neutrons in an atom of element X with atomic number 29 and mass number 63
Answer
Protons
The number of protons of element X is the same as the atomic number
Number of protons = 29
Electrons
The neutral atom of element X therefore also has 29 electrons
Neutrons
The atomic number of an element X atom is 29 and its mass number is 63
Number of neutrons = mass number – number of protons
Number of neutrons = 63 – 29
Number of neutrons = 34
Examiner Tips and Tricks
Both the atomic number and the relative atomic number (which you can use as the mass number) are given on the Periodic Table but it can be easy to confuse them.
Think MASS = MASSIVE, as the mass number is always the bigger of the two numbers, the other smaller one is thus the atomic / proton number.
Beware that some Periodic Tables show the numbers the other way round with the atomic number at the bottom!

Unlock more, it's free!
Did this page help you?