Identification of Cations (Cambridge (CIE) IGCSE Chemistry): Revision Note
Exam code: 0620 & 0971
Identification of cations
Test for ammonium ions
Ammonium ions, NH4+, can be identified by gently warming a solution containing the ions with sodium hydroxide solution
The sodium hydroxide solution is a source of hydroxide ions, OH–, for the test
This releases ammonia gas which turns damp red litmus paper blue
Testing for ammonium ions

Heating ammonium ions with sodium hydroxide solution releases ammonia gas which turns damp red litmus blue
Metal cations in aqueous solution can be identified by the colour of the precipitate they form on addition of sodium hydroxide and ammonia
Most transition metals produce hydroxides with distinctive colours
Test for metal ions with sodium hydroxide solution
If a small amount of sodium hydroxide solution is used, the resulting metal hydroxide normally precipitates out of solution
If excess sodium hydroxide solution is used, some of the precipitates may re-dissolve
For this reason, just a few drops of sodium hydroxide solution are added at first and very slowly
The sodium hydroxide test for the metal ion is:
Add a few drops of sodium hydroxide solution
Record any colour changes or precipitates formed
Add excess sodium hydroxide solution
Record any colour changes or changes to precipitates
Test with sodium hydroxide summary
Metal Ion | Effect of aqueous NaOH | Effect of excess aqueous NaOH |
|---|---|---|
aluminium, Al3+ | white precipitate | soluble in excess giving a colourless solution |
ammonium, NH4+ | ammonia produced on warming | - |
calcium, Ca2+ | white precipitate | insoluble in excess |
chromium(III), Cr3+ | green precipitate | soluble in excess |
copper, Cu2+ | light blue precipitate | insoluble in excess |
iron, Fe2+ | green precipitate | insoluble in excess, but the surface of the precipitate turns brown on standing |
iron, Fe3+ | red-brown precipitate | insoluble in excess |
zinc, Zn2+ | white precipitate | soluble in excess giving a colourless solution |
Test for metal ions with ammonia solution
If a small amount of ammonia solution is used, the resulting metal hydroxide normally precipitates out of solution
If excess ammonia solution is used, some of the precipitates may re-dissolve
For this reason, just a few drops of ammonia solution are added at first and very slowly
The ammonia test for the metal ion is:
Add a few drops of ammonia solution
Record any colour changes or precipitates formed
Add excess ammonia solution
Record any colour changes or changes to precipitates
Test with ammonia summary
Metal Ion | Addition of 2-3 drops of ammonia | Addition of excess ammonia |
|---|---|---|
aluminium, Al3+ | white precipitate | insoluble in excess |
calcium, Ca2+ | no precipitate or very slight white precipitate | no change |
chromium(III), Cr3+ | green precipitate | insoluble in excess |
copper, Cu2+ | light blue precipitate | soluble in excess giving a dark blue solution |
iron, Fe2+ | green precipitate | insoluble in excess, but the surface of the precipitate turns brown on standing |
iron, Fe3+ | red-brown precipitate | insoluble in excess |
zinc, Zn2+ | white precipitate | soluble in excess giving a colourless solution |
Analysing results
The tables above contain the results for all metal cations included in the syllabus
For example, zinc chloride:
ZnCl2 (aq) + 2NaOH (aq) → Zn(OH)2 (s) + 2NaCl (aq)
There are 3 metal ions that all form white precipitates:
Aluminium ions, Al3+
Calcium ions, Ca2+
Zinc ions, Zn2+
Calcium ions, Ca2+, can be easily distinguished from Zn2+ and Al3+
The white precipitate of calcium hydroxide does not dissolve in excess sodium hydroxide solution
The white precipitates of zinc hydroxide and aluminium hydroxide dissolve in excess sodium hydroxide solution
Zinc ions, Zn2+, can then be distinguished from Al3+ ions as
The white precipitate of zinc hydroxide dissolves in excess ammonia solution
The white precipitate of aluminium hydroxide does not dissolve in excess ammonia solution
Examiner Tips and Tricks
The ammonia or sodium hydroxide solution must be added very slowly. If it is added too quickly and the precipitate is soluble in excess, then you run the risk of missing the formation of the initial precipitate, which dissolves as quickly as it forms if excess solution is added.
Be sure to distinguish between the term “colourless” and “clear”. A solution that loses its colour has become colourless. A clear solution is one that you can see through such as water. Solutions can be clear and have colour eg. dilute copper sulphate.
Flame tests for metal ions
The flame test is used to identify the metal cations by the colour of the flame they produce
Ions from different metals produce different colours
Dip the loop of an unreactive metal wire such as nichrome or platinum in concentrated acid and then hold it in the blue flame of a Bunsen burner until there is no colour change
This is an important step as the test will only work if there is just one type of ion present
Two or more ions means the colours will mix, making identification erroneous
This cleans the wire loop and avoids contamination
A small sample of the compound is placed on an unreactive metal wire loop such as nichrome or platinum
Dip the loop into the solid sample / solution and place it in the edge of the blue Bunsen flame
Avoid letting the wire get so hot that it glows red otherwise this can be confused with a flame colour

Diagram showing the technique for carrying out a flame test
The colour of the flame is observed and used to identify the metal ion present:
Cation | Flame Colour |
Li+ | Red |
Na+ | Yellow |
K+ | Lilac |
Ca2+ | Orange-red |
Ba2+ | Light-green |
Cu2+ | Blue-green |
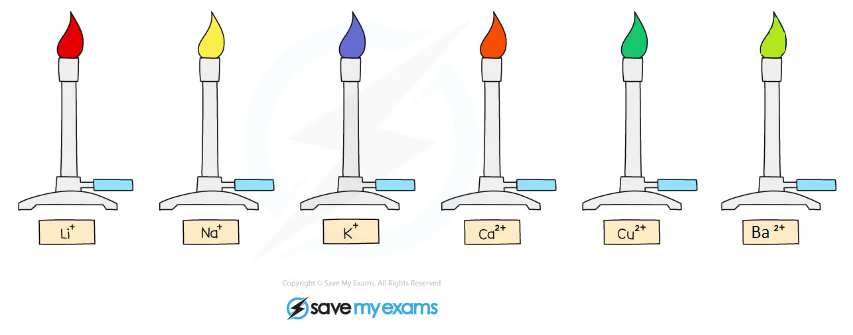
Metal ions form distinctive coloured flames
Examiner Tips and Tricks
The sample needs to be heated strongly, so the Bunsen burner flame should be on a blue flame.

Unlock more, it's free!
Did this page help you?