Acid-Base Titrations (Cambridge (CIE) IGCSE Chemistry): Revision Note
Exam code: 0620 & 0971
Acid-base titrations
Titrations are a method of analysing the concentration of solutions
They can determine exactly how much alkali is needed to neutralise a quantity of acid – and vice versa
You may be asked to perform titration calculations to determine the moles present in a given amount or the concentration / volume required to neutralise an acid or a base
Titrations can also be used to prepare salts
Apparatus
25 cm3 volumetric pipette
Pipette filler
50 cm3 burette
250 cm3 conical flask
Small funnel
0.1 mol / dm3 sodium hydroxide solution
Sulfuric acid of unknown concentration
A suitable indicator
Clamp stand, clamp & white tile
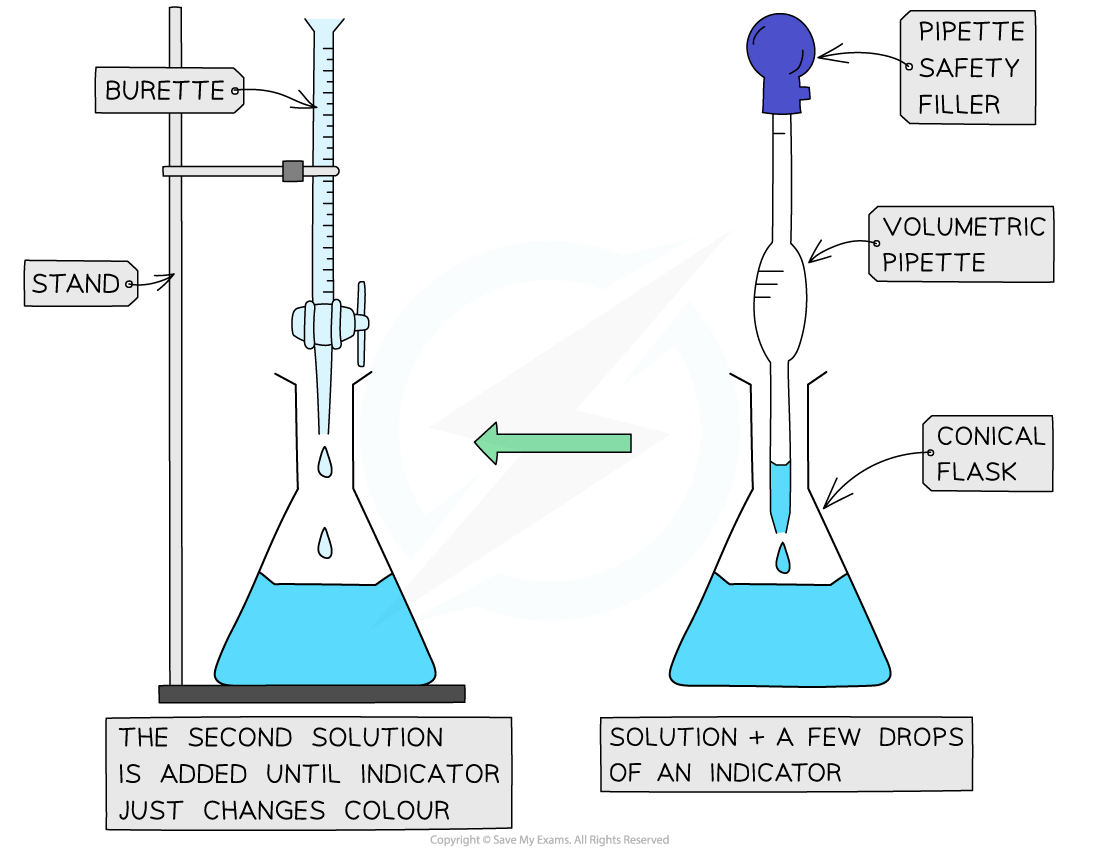
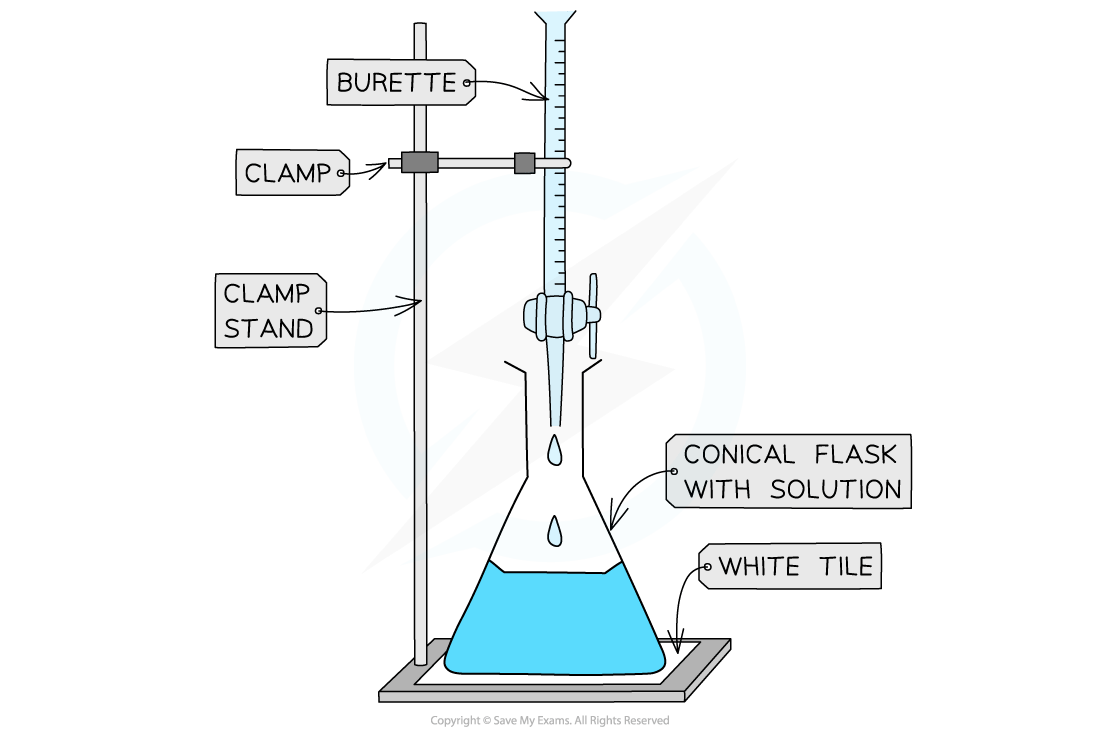
The steps in performing a titration
Method
Use the pipette and pipette filler and place exactly 25 cm3 sodium hydroxide solution into the conical flask
Using the funnel, fill the burette with hydrochloric acid placing an empty beaker underneath the tap. Run a small portion of acid through the burette to remove any air bubbles
Record the starting point on the burette to the nearest 0.05 cm3
Place the conical flask on a white tile so the tip of the burette is inside the flask
Add a few drops of a suitable indicator to the solution in the conical flask
Perform a rough titration by taking the burette reading and running in the solution in 1 – 3 cm3 portions, while swirling the flask vigorously
Quickly close the tap when the end-point is reached
The endpoint is when one drop causes a sharp colour change
Record the volume of hydrochloric acid added, in a suitable results table as shown below
Make sure your eye is level with the meniscus
Repeat the titration with a fresh batch of sodium hydroxide
As the rough end-point volume is approached, add the solution from the burette one drop at a time until the indicator just changes colour
Record the volume to the nearest 0.05 cm3
Repeat until you achieve two concordant results (two results that are within 0.1 cm3 of each other) to increase accuracy
| Rough titre | Titre 1 | Titre 2 | Titre 3 |
Final reading (cm3) |
|
|
|
|
First reading (cm3) |
|
|
|
|
Titre (cm3) |
|
|
|
|
Examiner Tips and Tricks
Common errors during a titration include:
Not removing the funnel from the burette
This can lead to some liquid dripping into the burette and cause false / high readings
Not filling the jet space of the burette
The jet space is the part of the burette after the tap
Not filling this space can lead to false readings
Reading the volume from the burette incorrectly
Readings should be taken from the bottom of the meniscus
Careful: The scale on the burette has 0.0 cm3 at the top and 50 cm3 (typically) at the bottom
Indicators
Indicators are used to show the endpoint in a titration
Wide range indicators such as litmus are not suitable for titration as they do not give a sharp colour change at the endpoint
However, methyl orange and phenolphthalein are very suitable
Some of the most common indicators with their corresponding colours are shown below:
Common acid-base indicators
Indicator | Colour in acid | Colour in alkali | Colour in neutral |
|---|---|---|---|
Litmus solution | Red | Blue | Purple |
Red litmus paper | Stays red | Turns blue | No change |
Blue litmus paper | Turns red | Stays blue | No change |
Methyl orange | Red | Yellow | Orange |
Phenolphthalein | Colourless | Pink | Colourless |
Thymolphthalein | Colourless | Blue | Colourless |

Unlock more, it's free!
Did this page help you?