Apparatus for Measurements (Cambridge (CIE) IGCSE Chemistry): Revision Note
Exam code: 0620 & 0971
Time, temperature, mass & volume
Time
Time can be measured using a stopwatch or stopclock which are usually accurate to one or two decimal places
The units of time normally used are seconds or minutes
Other units may be used for extremely slow reactions (e.g. rusting)
Remember: 1 minute = 60 seconds
Examiner Tips and Tricks
Careful: Units of time often cause issues in results tables.
If the display on a stopwatch showed 1:30.
The incorrect time to record would be 1.30 minutes.
The correct time would be 1.5 minutes.
To avoid any confusion, if the time intervals are less than a minute, it is best / easier to change the recorded units to seconds.
So, the same stopwatch display would be recorded as 90 seconds.
Temperature
Temperature is measured with a thermometer or digital temperature probe
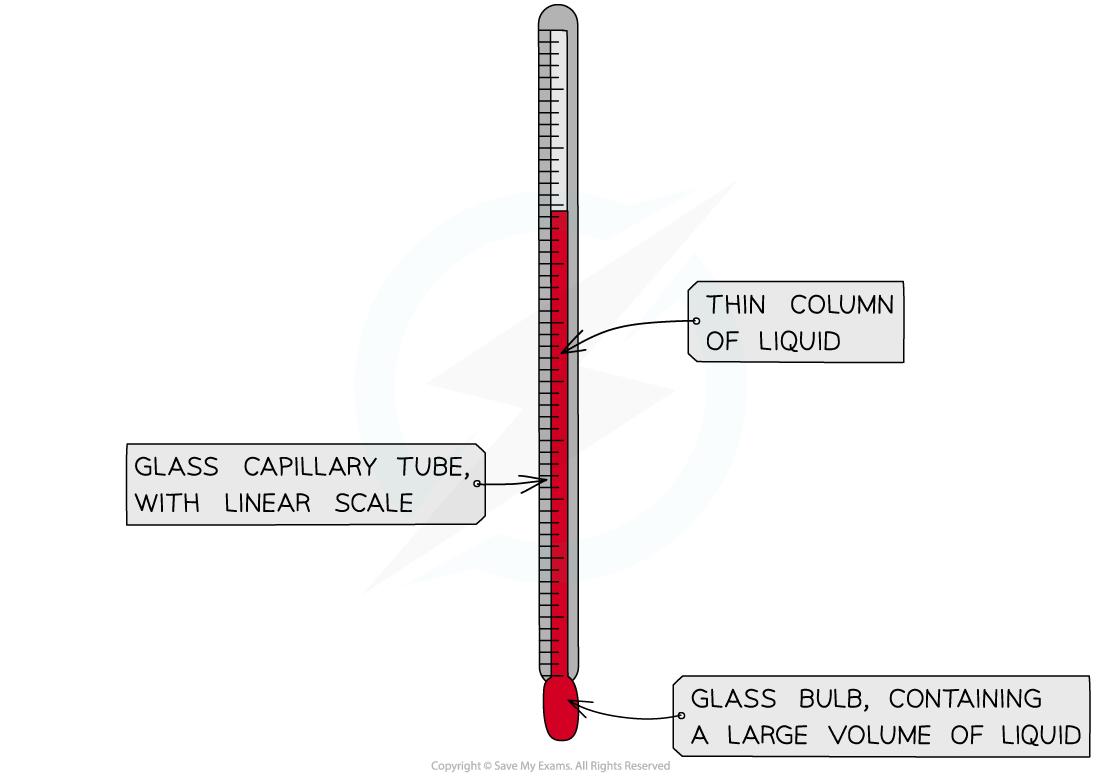 |  |
Laboratory thermometers usually have a precision of a half or one degree
Digital temperature probes are available which are more precise than traditional thermometers and can often read to 0.1 oC
Traditional thermometers rely upon the uniform expansion and contraction of a liquid substance with temperature
Digital temperature probes can be just as, if not, more accurate than traditional thermometers
The units of temperature are degrees Celsius (ºC)
Mass
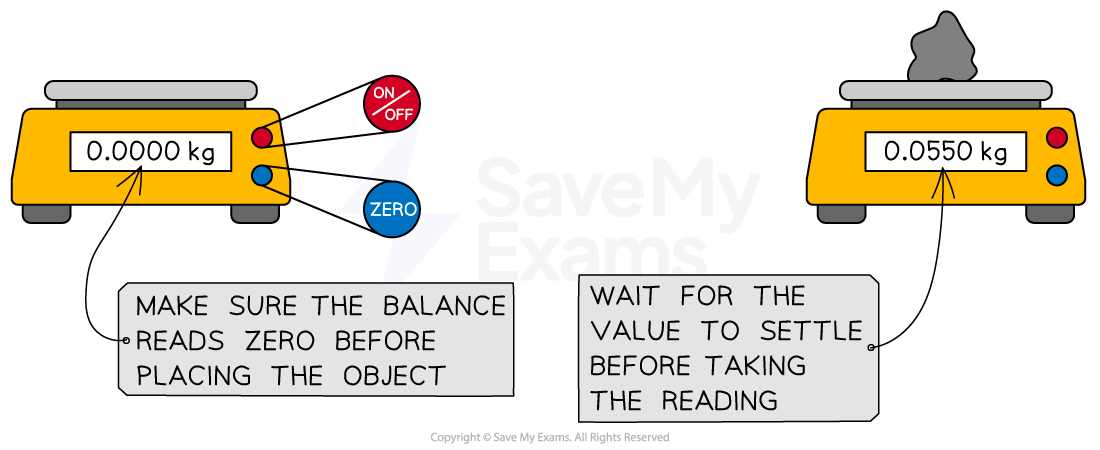
Mass is measured using a digital balance which normally gives readings to two decimal places
Balances should be tared (set to zero) before use
Balances should also be allowed time to settle on a final measurement / reading before it is recorded
The standard unit of mass in kilograms (kg)
However, in chemistry grams (g) are most often used
Remember: 1 kilogram = 1000 grams
Volumes of liquid
The volume of a liquid can be determined using different pieces of apparatus
The choice of apparatus depends on the level of accuracy needed
Three common pieces of apparatus for measuring the volume of a liquid are:
Burettes
Volumetric pipettes
Measuring cylinders
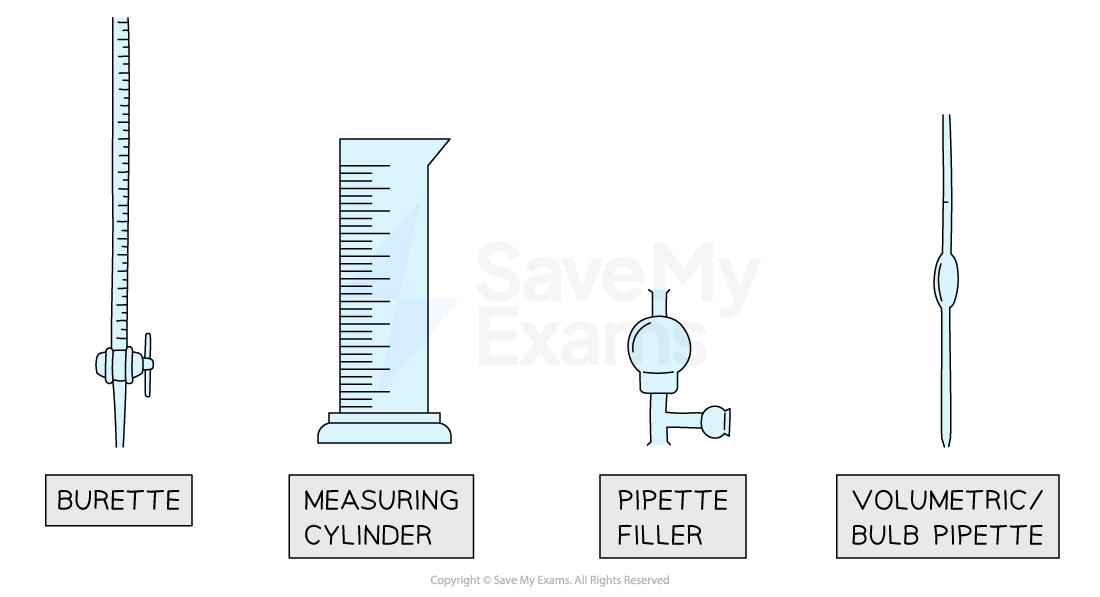
Burettes are the most accurate way of measuring a variable volume of liquid between 0 cm3 and 50 cm3
They are most commonly used in titrations
Careful: Read the burette scale from top to bottom as 0.00 cm3 is at the top of the column
Volumetric pipettes are the most accurate way of measuring a fixed volume of liquid,
They have a scratch mark on the neck which is matched to the bottom of the meniscus to make the measurement
A pipette filler is used to draw the liquid into the volumetric pipette
The most common volumes for volumetric pipettes are 10 cm3 and 25 cm3
Measuring cylinders are used when approximate volumes are required (accuracy is not an important factor)
These are graduated (have a scale so can be used to measure)
Measuring cylinders typically range from 10 cm3 to 1 litre (1 dm3)
Whichever apparatus you use, you may see markings in millilitres, ml, which are the same as a cm3
Volumes of gas
For some experiments, the volume of a gas produced needs to be measured
This is typically done by using one of the following methods:
Using a gas syringe
By downward displacement of water
A gas syringe is more precise and accurate than downward displacement of water
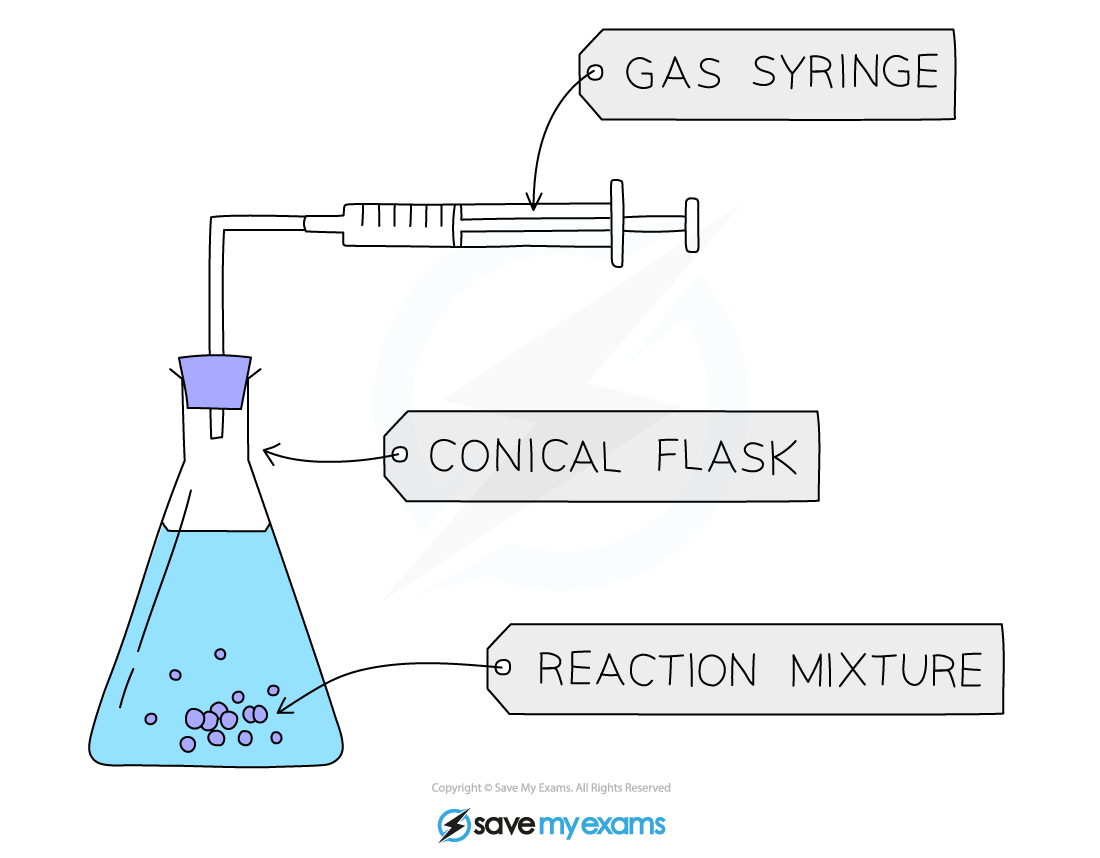
Diagram of the set-up for an experiment involving a gas syringe
Downward displacement of water is where a measuring cylinder is inverted in water to collect the gas produced
This method does not work if the gas is soluble in water
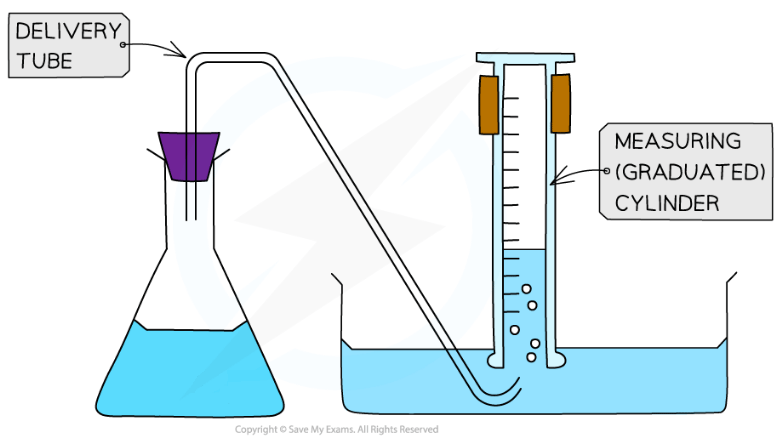
Diagram of the set-up for an experiment collecting gas by downward displacement of water
If the gas happens to be heavier than air and is coloured, the cylinder does not need to be inverted
Advantages & disadvantages of methods & apparatus
In the lab, we often have choices of different apparatus to do the same job
Evaluating which piece of apparatus is the best one to use is part of good experimental planning and design
This means appreciating some of the advantages and disadvantages of laboratory apparatus
Advantages and disadvantages of lab apparatus
Apparatus | Advantage | Disadvantage |
|---|---|---|
Temperature probe |
|
|
Volumetric pipette |
|
|
Gas syringe |
|
|
Microscale experiments |
|
|
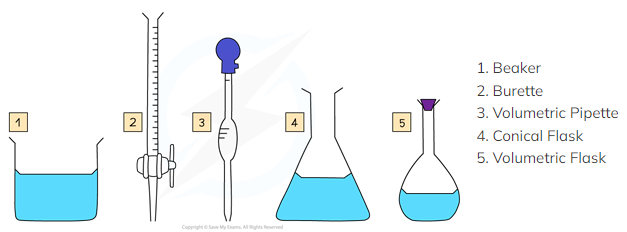
Five pieces of apparatus that can be used to measure the volume of a liquid. They all have their pros and cons
Planning your method
Good experimental design includes the answers to questions like
Have I chosen a suitable apparatus for what I need to measure?
Is it going to give me results in an appropriate time frame?
Is it going to give me enough results to process, analyse and make conclusions?
Does it allow for repetitions to check how reliable my results are?
Does my plan give a suitable range of results?
How can I be sure my results are accurate?
Have I chosen an appropriate scale of quantities without being wasteful or unsafe?
You may be asked about experimental methods in exam questions and your experience and knowledge of practical techniques in chemistry should help you to spot mistakes and suggest improvements
Examiner Tips and Tricks
Make sure you know the names of common laboratory apparatus.

Unlock more, it's free!
Did this page help you?