Alkenes (Cambridge (CIE) IGCSE Chemistry): Revision Note
Exam code: 0620 & 0971
Did this video help you?
Catalytic cracking
Alkenes are unsaturated hydrocarbons with carbon-carbon double bonds (C=C)
They have covalent bonds
Their general formula is CnH2n
The presence of the double bond, C=C, means they can make more bonds with other atoms by opening up the C=C bond and allowing incoming atoms to form another single bond with each carbon atom of the functional group
Each of these carbon atoms now forms 4 single bonds instead of 1 double and 2 single bonds
This makes them much more reactive than alkanes
Table of alkenes
Displayed formula | Name | Molecular formula |
|---|---|---|
 | ethene | C2H4 |
 | propene | C3H6 |
 | but-1-ene | C4H8 |
 | pent-1-ene | C5H10 |
The first four members of the alkene homologous series
Manufacture of alkenes
Although there is use for each fraction obtained from the fractional distillation of crude oil, the amount of longer chain hydrocarbons produced is far greater than needed
These long chain hydrocarbon molecules are further processed to produce other products
A process called catalytic cracking is used to convert longer-chain molecules into short-chain and more useful hydrocarbons
Shorter chain alkanes, alkenes and hydrogen are produced from the cracking of longer chain alkanes
Alkenes can be used to make polymers and the hydrogen used to make ammonia
Kerosene and diesel oil are often cracked to produce petrol, other alkenes and hydrogen
Cracking involves heating the hydrocarbon molecules to around 600 – 700°C to vaporise them
The vapours then pass over a hot powdered catalyst of alumina or silica
This process breaks covalent bonds in the molecules as they come into contact with the surface of the catalyst, causing thermal decomposition reactions
The molecules are broken up in a random way which produces a mixture of smaller alkanes and alkenes
Hydrogen and a higher proportion of alkenes are formed at higher temperatures and higher pressure
The cracking of decane
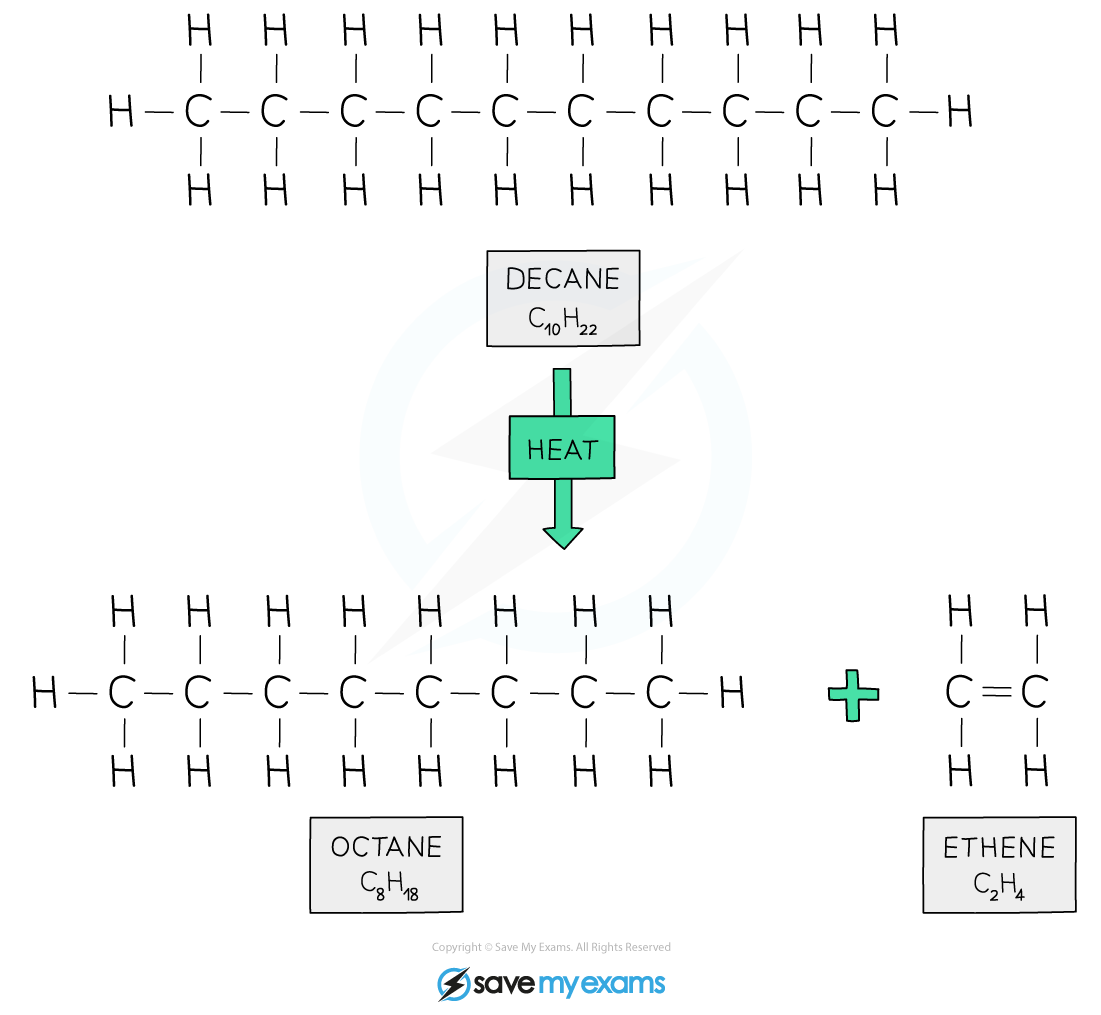
Decane is catalytically cracked to produce octane for petrol and ethene for ethanol
Distinguishing from alkanes
The presence of the C=C double bond in an alkene but not an alkane allows us to distinguish between them
To do this, bromine water is used
Bromine water is an orange coloured solution
When bromine water is shaken with an alkane the solution remains orange
When bromine water is shaken with an alkene, the solution will go colourless, as the bromine can add across the double bond meaning it is no longer in solution
How to distinguish between alkanes and alkenes
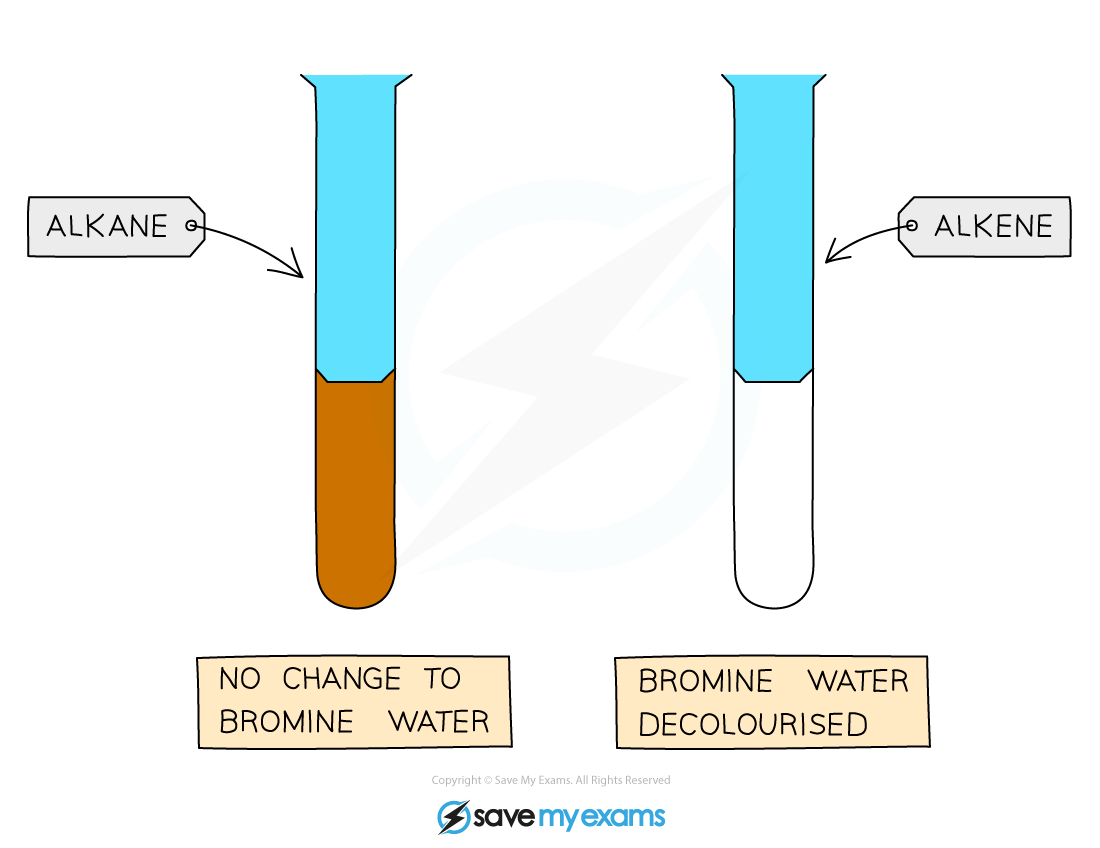
Alkenes will decolourise bromine water
Examiner Tips and Tricks
When describing what happens to bromine water in an alkene ensure you say colourless, and not clear.

Unlock more, it's free!
Did this page help you?