Naming Organic Compounds (Cambridge (CIE) IGCSE Chemistry): Revision Note
Exam code: 0620 & 0971
Naming organic compounds
The names of organic compounds have two main parts:
the stem (sometimes called the prefix)
end part (or suffix)
The stem indicates the number of carbon atoms present in the longest continuous chain of the compound
Number of carbon atoms in the longest chain | Part of the chemical's name |
|---|---|
1 | meth |
2 | eth |
3 | prop |
4 | but |
5 | pent |
6 | hex |
The end part of the name tells you what functional group is in the compound
End part of the name | Functional group | Organic family |
|---|---|---|
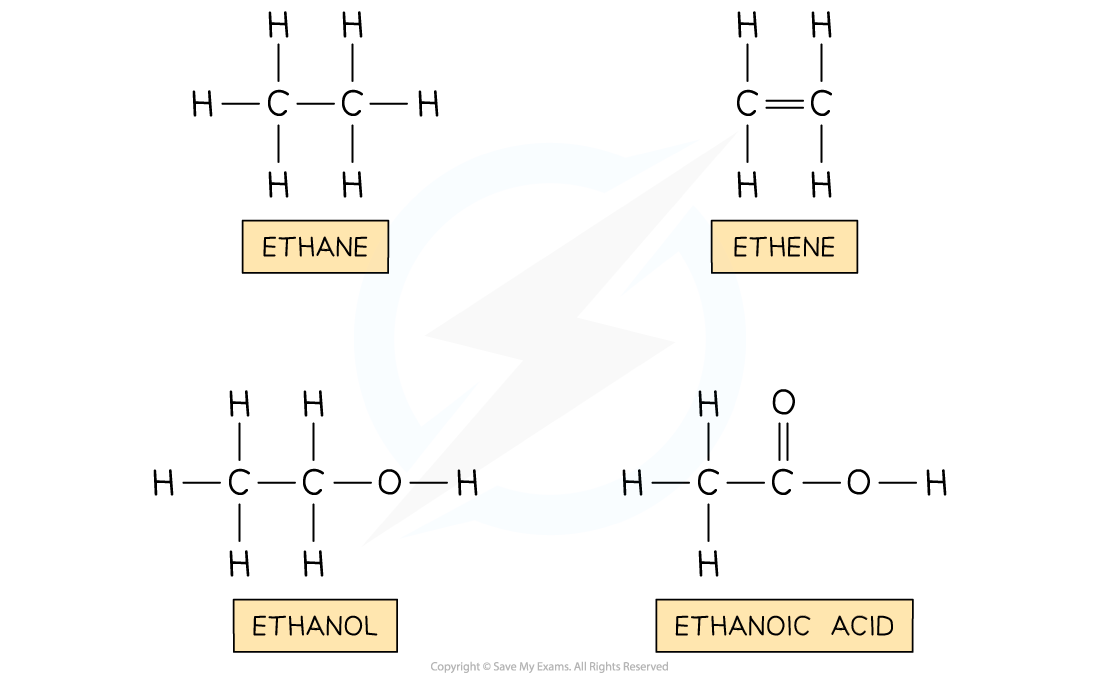
ane | none | Alkane |
ene | C=C bond | Alkene |
anol | –OH | Alcohol |
anoic acid | –COOH | Carboxylic acid |
amine | –NH2 | Amine |
-yl -anoate | –COO– | Ester |
Structures of organic compounds
Worked Example
Name the following organic compounds:
1 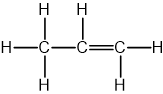 | 2 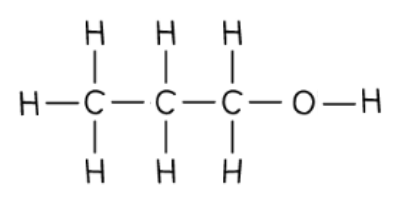 | 3 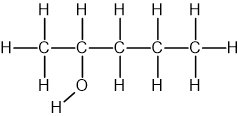 |
Answers:
Propene
The longest carbon chain is 3 carbons, so the name contains prop
The functional group is C=C, so the name contains -ene
Propanol
The longest chain is 3 carbons, so the name is prop
The functional group is OH, so the name contains -anol
Pentanol
The longest carbon chain is 5 carbons, so the name contains pent
The functional group is OH, so the name contains -anol
Examiner Tips and Tricks
Make sure you can draw and name the structures given above.
Further naming of organic compounds
Extended tier only
When there is more than one carbon atom where a functional group can be located it is important to distinguish exactly which carbon the functional group is on
Each carbon is numbered and these numbers are used to describe where the functional group is
For example:
Propan-1-ol is an alcohol with an -OH functional group
The 1 in the name indicates that the -OH group is located on the first carbon atom
Careful: There are many times when the numbering should start from the right - this is to keep the numbers as low as possible in chemical names
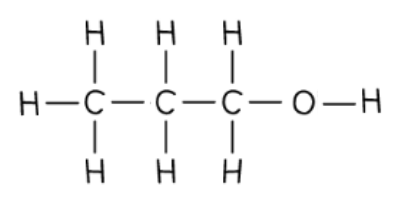
In propan-1-ol the -OH group is located on the first carbon atom
Alkanes
As before, the number of carbon atoms gives the part of the compounds name
Alkanes contain only single carbon-carbon bonds, C–C, which means that their name ends with –ane
Some compounds, like alcohols and carboxylic acids, have –an– in their name
This indicates that those compounds contain only single carbon-carbon bonds, C–C
Alkenes
As before, the number of carbon atoms gives the part of the compounds name
Alkenes contain at least one double carbon-carbon bond, C=C, which means that their name ends with –ene
The first alkene is ethene because you must have two carbons to be able to form a double carbon-carbon bond, C=C
After propene, you must state the number of the first carbon that is part of the double carbon-carbon bond, C=C
e.g. but-1-ene has a double carbon-carbon bond, C=C, on the first carbon in the chain
But-2-ene has a double carbon-carbon bond, C=C, on the second carbon in the chain
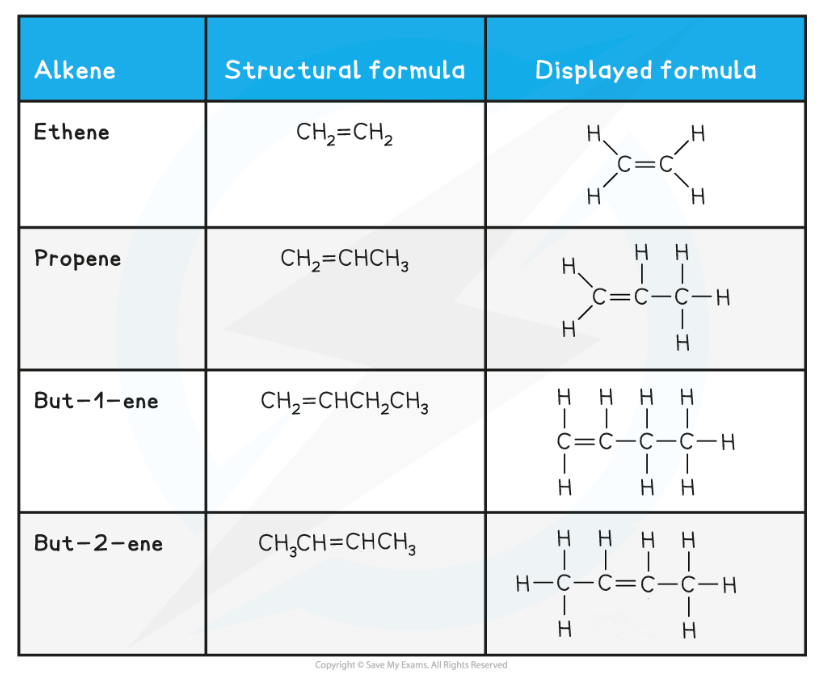
Alcohols
As before, the number of carbon atoms gives the part of the compounds name
Alcohols contain an alcohol / hydroxyl group, –O–H, which means that their name ends with –anol
After ethanol, you must state the number of the carbon that has the alcohol / hydroxyl group, –O–H, attached
e.g. propan-1-ol has the alcohol / hydroxyl group, –O–H, on the first carbon in the chain
Propan-2-ol has the alcohol / hydroxyl group, –O–H, on the second carbon in the chain
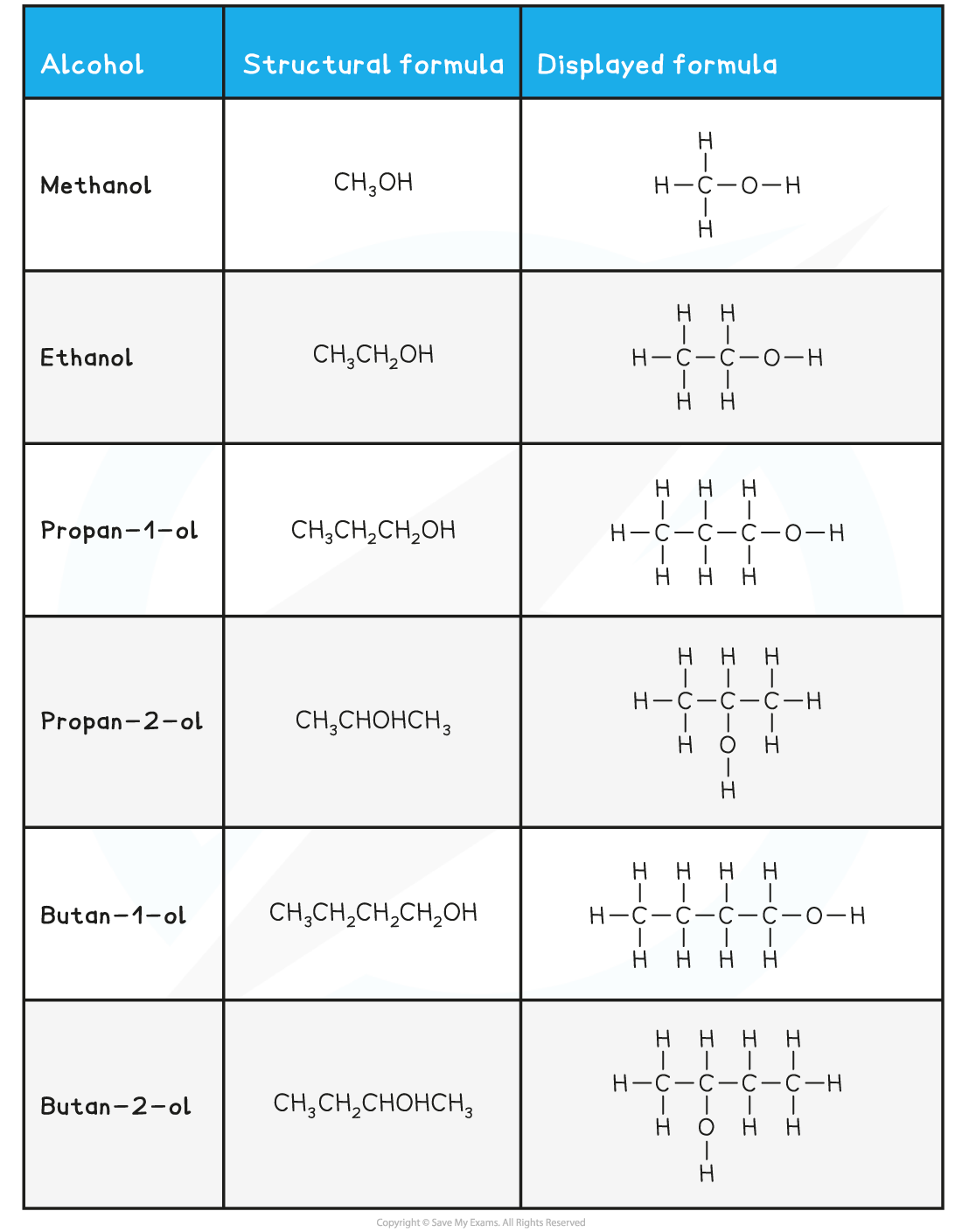
Carboxylic acids
As before, the number of carbon atoms gives the part of the compounds name
Carboxylic acids contain a carboxylic acid group, –COOH, which means that their name ends with –anoic acid
There is no need to number carboxylic acids because the carbon that is part of the carboxylic acid group is automatically the first carbon of the chain
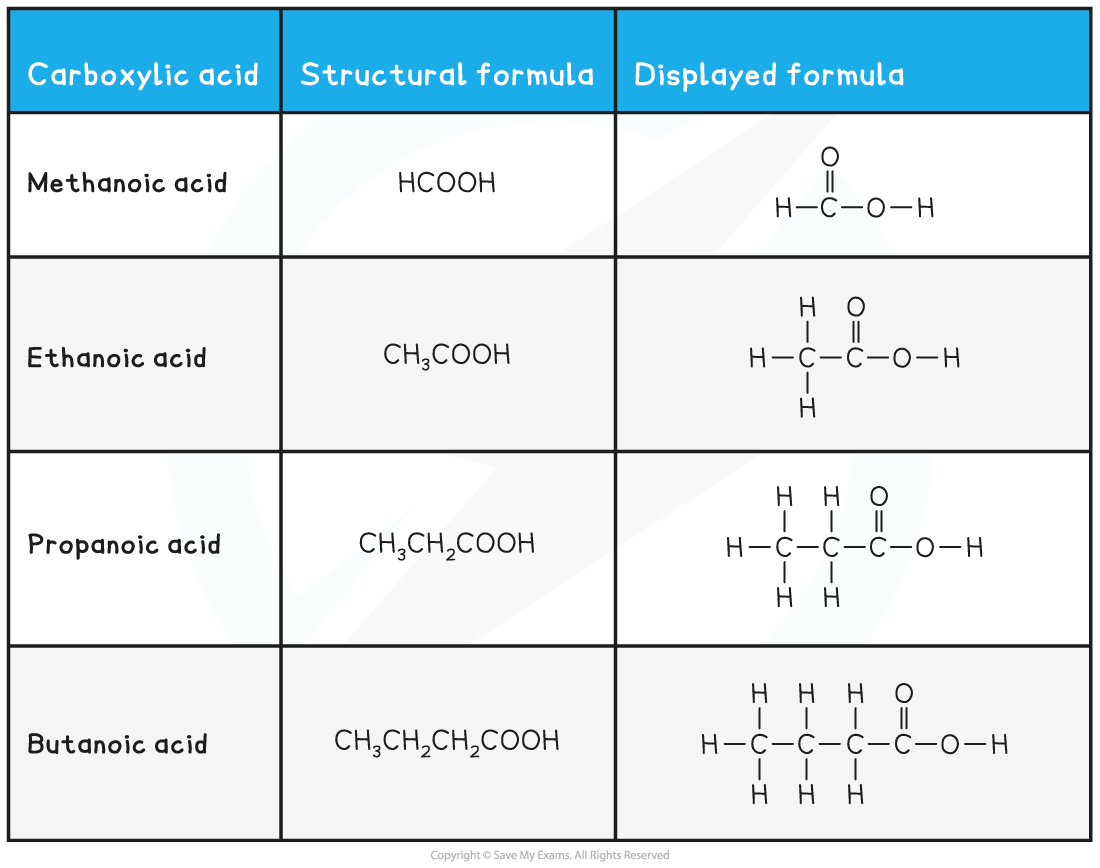
Esters
Esters are one of the more challenging compounds to name
Their name is based on the original alcohol and carboxylic acid that they were prepared from
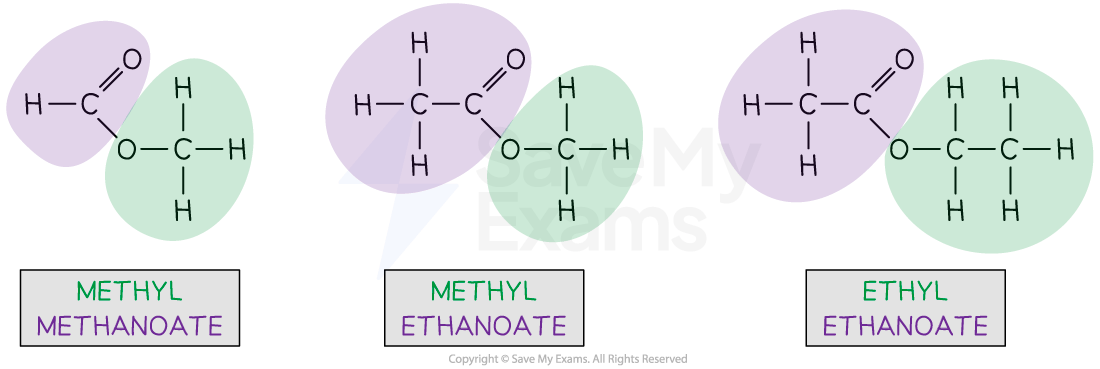
Ester names are confusing because the name is written backwards from the way the structure is drawn

Methyl methanoate
The alcohol portion of the molecule contains the C–O single bond and is coloured green
There is one carbon, so this gives the methyl part of the name
The carboxylic acid portion contains the C=O double bond and is coloured purple
The is one carbon, so this gives the methanoate part of the name
Methyl ethanoate
The alcohol portion of the molecule contains the C–O single bond and is coloured green
There is one carbon, so this gives the methyl part of the name
The carboxylic acid portion contains the C=O double bond and is coloured purple
The are two carbons, so this gives the ethanoate part of the name
Ethyl ethanoate
The alcohol portion of the molecule contains the C–O single bond and is coloured green
There are two carbons, so this gives the ethyl part of the name
The carboxylic acid portion contains the C=O double bond and is coloured purple
The are two carbons, so this gives the ethanoate part of the name
Examiner Tips and Tricks
Extended tier students should be able to draw the structural and displayed formulae for all of the compounds written above.

Unlock more, it's free!
Did this page help you?