States of Matter (Cambridge (CIE) IGCSE Chemistry): Revision Note
Exam code: 0620 & 0971
Did this video help you?
State changes
State changes occur when:
Solids become liquids
Liquids become gases
Gases become liquids
Liquids become solids
Each state change requires a change in the energy, arrangement and movement of the particles
The state changes

The inter-conversions / state changes are shown in relation to energy
Melting
Melting is when a solid changes into a liquid
Requires heat energy which transforms into kinetic energy, allowing the particles to move
Occurs at a specific temperature known as the melting point (m.p.)
Freezing
Freezing is when a liquid changes into a solid
This is the reverse of melting and occurs at exactly the same temperature as melting, hence the melting point and freezing point of a pure substance are the same. Water, for example, freezes and melts at 0 ºC
Requires a significant decrease in temperature (or loss of thermal energy) and occurs at a specific temperature
Boiling
Boiling is when a liquid changes into a gas
Requires heat which causes bubbles of gas to form below the surface of a liquid, allowing for liquid particles to escape from the surface and within the liquid
Occurs at a specific temperature known as the boiling point (b.p.)
Evaporation
Evaporation occurs when a liquid changes into a gas and occurs over a range of temperatures
Evaporation occurs only at the surface of liquids where high energy particles can escape from the liquid's surface at low temperatures, below the b.p. of the liquid
The larger the surface area and the warmer the liquid surface, the more quickly a liquid can evaporate
Condensation
Condensation occurs when a gas changes into a liquid on cooling and it takes place over a range of temperatures
When a gas is cooled its particles lose energy and when they bump into each other they lack the energy to bounce away again, instead they group together to form a liquid
Examiner Tips and Tricks
Questions on the particle theory of matter show interconversion of states with a reversible arrow: ⇌, which means that the process can go forwards and backwards.
Read the question carefully and pick the direction of the change in state that the question refers to.
Did this video help you?
State changes & kinetic theory
Extended tier only
When substances are heated, the particles absorb thermal energy which is converted into kinetic energy
This is the basis of the kinetic theory of matter
Heating a solid causes its particles to vibrate more
As the temperature increases, the particles vibrate so much that the solid expands until the structure breaks
This is when the solid melts into a liquid
Heating a liquid causes its particles to move more and spread out
Some particles at the surface gain sufficient energy to overcome the intermolecular forces
This is when a liquid starts to evaporate
When the boiling point is reached, all of the particles gain enough energy to escape and the liquids boils into a gas
These changes in state can be shown on a graph called a heating curve:
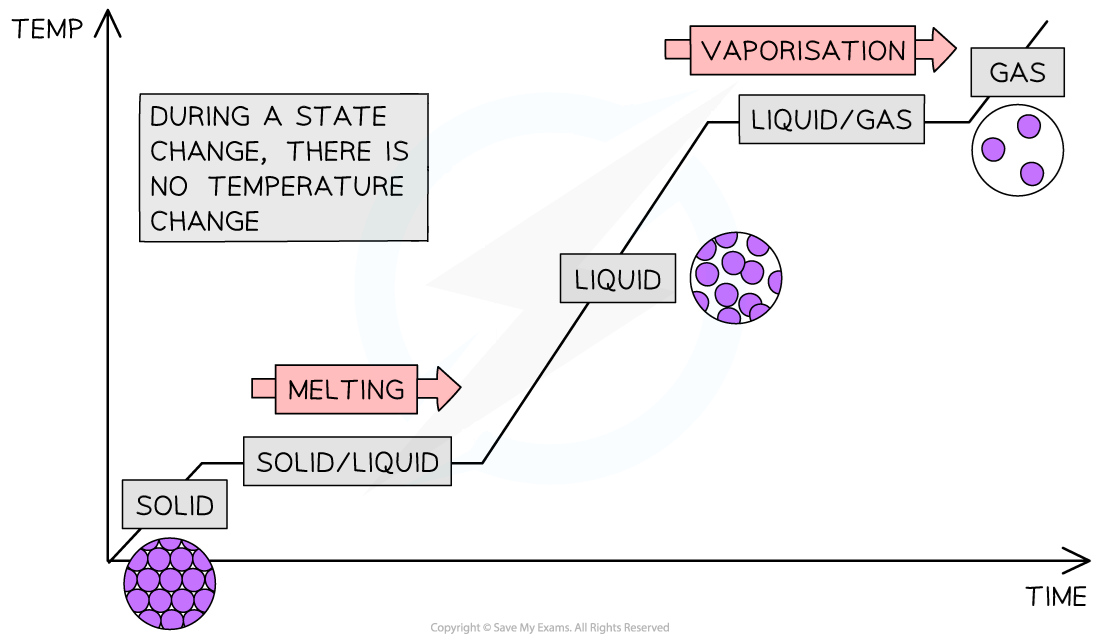
Cooling down a gas has the reverse effect and this would be called a cooling curve:

A cooling curve is like a heating curve, but is the mirror image
Heating and cooling curves are used to show how changes in temperature affect changes of state
The horizontal sections occur when there is a change of state but there is no change in temperature

Unlock more, it's free!
Did this page help you?