Isotopes - IGCSE Physics Definition
Reviewed by: Leander Oates
Last updated
What are isotopes?
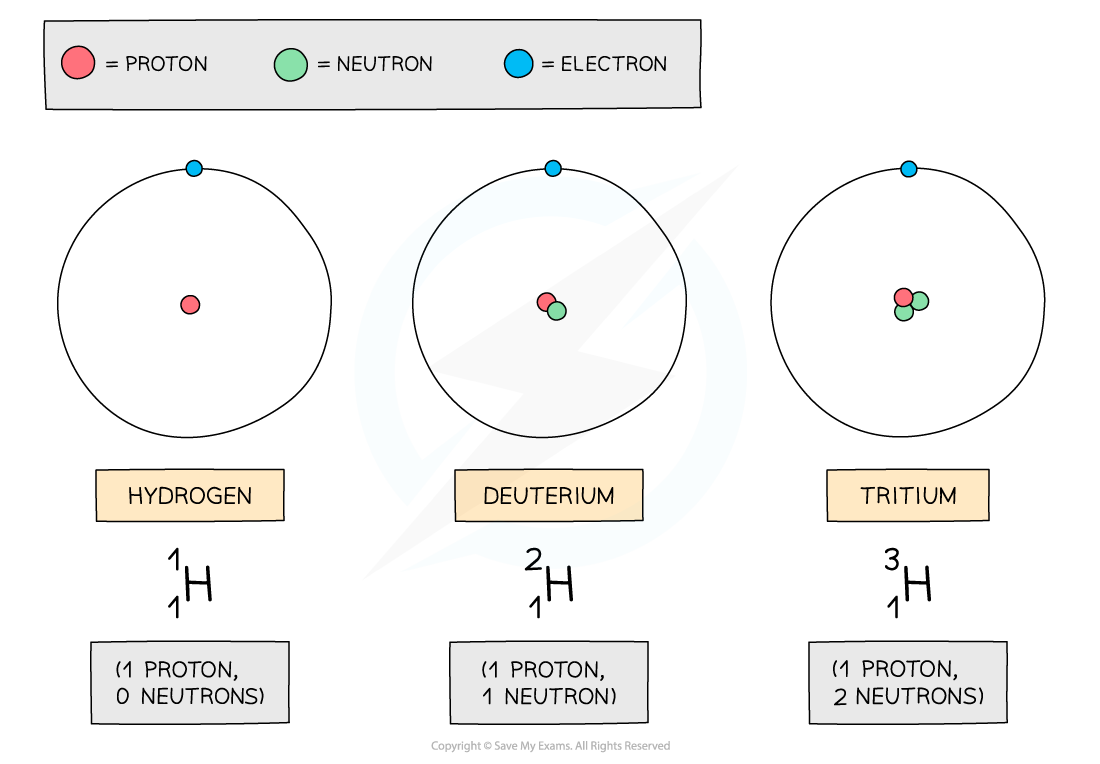
In IGCSE Physics, isotopes are different forms of the same chemical element that have the same number of protons but a different number of neutrons in their nuclei.
Isotopes of the same element have the same atomic number but different mass numbers, giving them slightly different physical properties while maintaining the same chemical properties.
Examiner-written IGCSE Physics revision resources that improve your grades 2x
- Written by expert teachers and examiners
- Aligned to exam specifications
- Everything you need to know, and nothing you don’t

Share this article


 written revision resources that improve your
written revision resources that improve your