Calculating Half-Life (WJEC GCSE Science (Double Award)): Revision Note
Exam code: 3430
Calculating Half-Life
Aside from calculating the half-life of an isotope, calculations involving half-life might involve
Predicting the amount remaining, or activity, of a sample after a certain time
Calculating the age of a sample
Predicting the amount of sample remaining
After each half-life, the amount of isotope remaining decreases by half
Half-life can also be represented in a table
As the number of half-lives increases, the proportion of the isotope remaining halves
Number of half-lives vs proportion of isotope remaining
Number of half-lives | Proportion of isotope remaining |
|---|---|
0 | 1 |
1 | |
2 | |
3 | |
4 |
Calculating the age of a sample
Carbon-14 can be used to estimate the age of organic samples in a process called carbon dating
This is done by measuring the proportion of carbon-14 to the proportion of the stable isotope carbon-12
The proportion of carbon-14 is constant in living organisms as carbon is continuously replaced during the period they are alive
When an organism dies, the activity of the carbon-14 begins to fall
So, the amount of carbon-14 remaining in a sample can be compared to the amount a living tissue contains to determine the approximate age of the sample
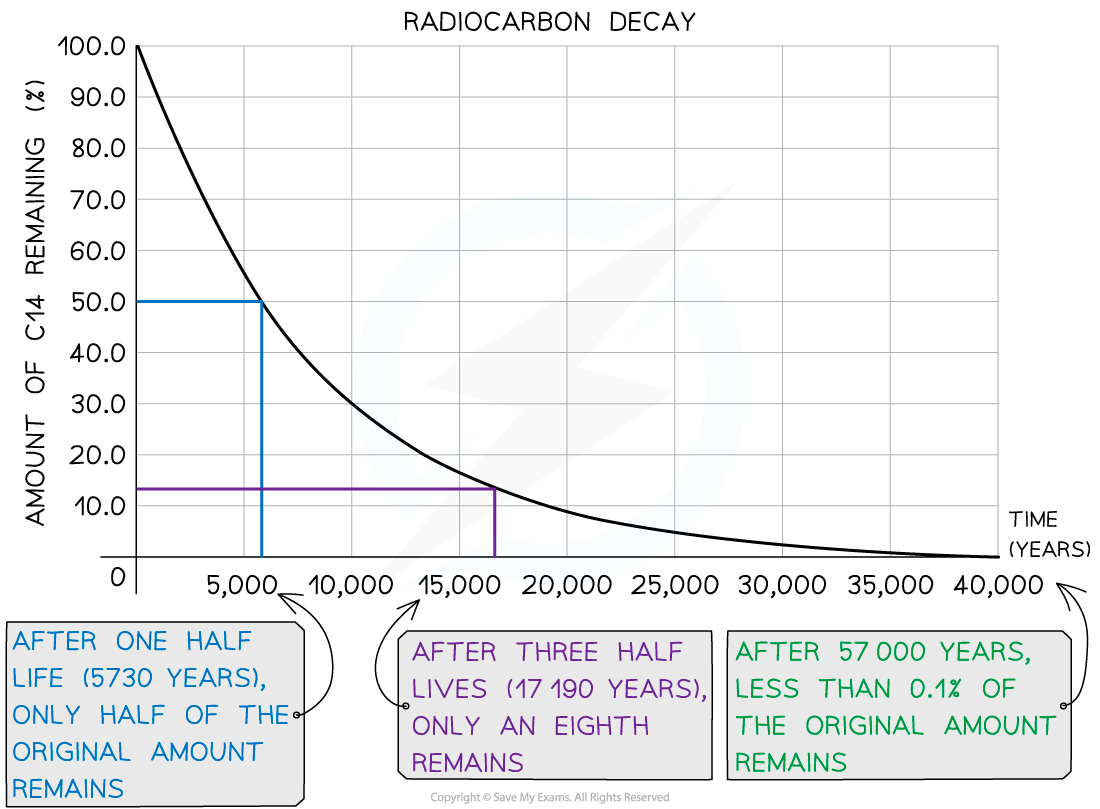
Carbon-14 has a half-life of around 5700 years
After 5700 years, there would be 50% of the original amount of carbon-14 remaining
After two half-lives, or 11 400 years, there would be just 25% of the carbon-14 remaining
Decay Curve for Carbon-14
Worked Example
A particular radioactive sample contains 2 million undecayed atoms. After a year, there are 500 000 atoms left undecayed.
Calculate the half-life of this sample.
Answer:
Step 1: Calculate how many times the number of un-decayed atoms has halved
There were 2 000 000 atoms to start with
1 000 000 atoms would remain after 1 half-life
500 000 atoms would remain after 2 half-lives
Therefore, the sample has undergone 2 half-lives
Step 2: Divide the time period by the number of half-lives
The time period is a year
The number of half-lives is 2
So two half-lives is 1 year, and one half-life is 6 months
Therefore, the half-life of the sample is 6 months
Worked Example
Carbon-14 has a half-life of 5730 years. A sample of ancient wood is found to have 12.5% of the carbon-14 as a living sample.
Estimate the age of the sample of ancient wood.
Answer:
Step 1: Determine the number of half-lives that have passed
After each half-life, the amount of carbon-14 remaining halves
Therefore, 12.5% remaining means that 3 half-lives have passed
Step 2: Determine the age of the sample
After 3 half-lives, the age of the sample is
age = 3 × 5730 = 17 190 years old

Unlock more, it's free!
Did this page help you?