Isotopes (AQA GCSE Combined Science: Trilogy): Revision Note
Exam code: 8464
Did this video help you?
Isotopes
Although the number of protons in a particular element is always the same, the number of neutrons can be different
Isotopes are atoms of the same element that have an equal number of protons but a different number of neutrons
In the diagram below are three isotopes of Hydrogen:
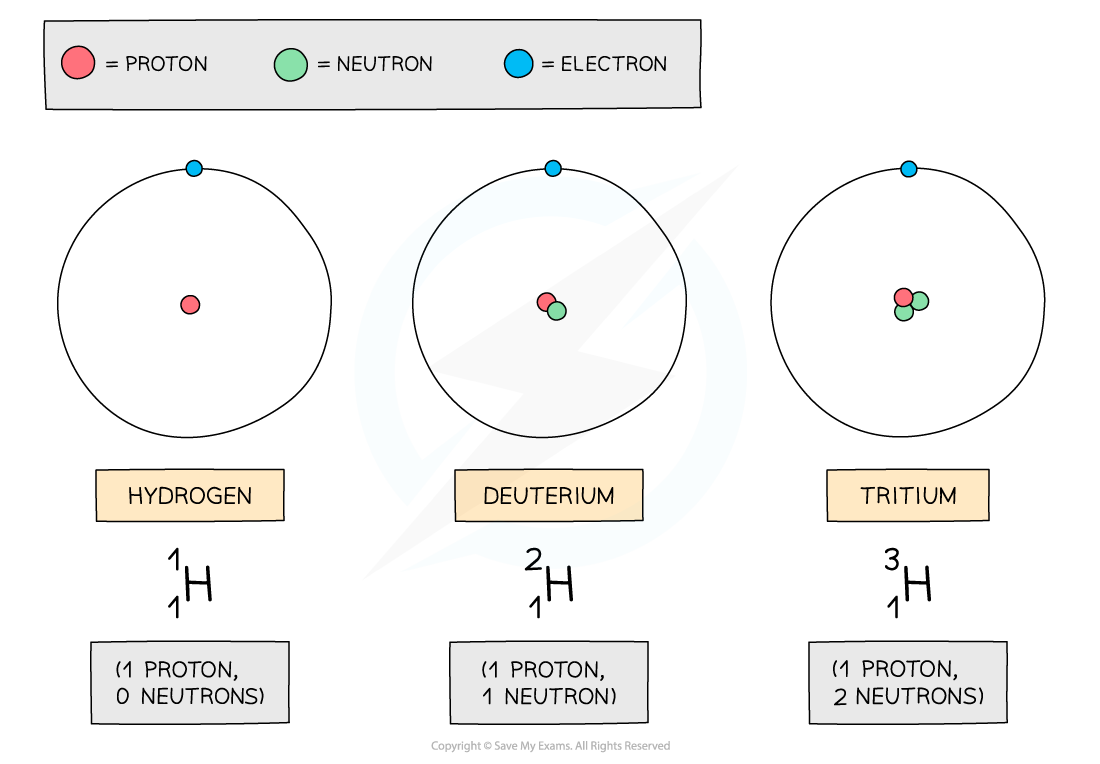
Hydrogen has three isotopes, each with a different number of neutrons
Isotopes occur naturally, but some are more rare than others
For example, about 2 in every 10,000 Hydrogen atoms is Deuterium
Tritium is even more rare (about 1 in every billion billion hydrogen atoms)
Examiner Tips and Tricks
This topic is also covered in Chemistry, although some of the terminologies may be a little different. However, in Physics you must refer to neutrons when explaining isotopes.
Differences Between Isotopes
The number of neutrons in an atom does not affect the chemical properties of an atom, such as its charge, but only its mass
This is because neutrons have no charge but do have mass
In the periodic table, the mass number of chlorine is often given as 35.5

This section of a periodic table shows chlorine as having a mass number of 35.5, but other elements have an integer mass number
The mass number of chlorine is given as 35.5 because it has 2 isotopes, one with a mass number of 35 and the other with a mass number of 37
Chlorine-35 is about three times more abundant than chlorine-37, so the given mass number of chlorine is closer to 35 than 37
The number of electrons and protons in different isotopes remains the same
Some isotopes are unstable as they have an imbalance of protons and neutrons
Worked Example
State the number of protons, neutrons and electrons in chlorine-35 and chlorine-37 atoms.
Answer:
Step 1: Determine the number of protons
The atomic number is the number of protons
Both Chlorine-35 and Chlorine-37 have 17 protons
Step 2: Determine the number of neutrons
The mass number is the number of protons and neutrons
Chlorine-35 neutrons: 35 - 17 = 18 neutrons
Chlorine-37 neutrons: 37 - 17 = 20 neutrons
Step 3: Determine the number of electrons
The number of electrons is equal to the number of protons
Both chlorine-35 and chlorine-37 have 17 electrons

Unlock more, it's free!
Did this page help you?