Specific Latent Heat (AQA GCSE Combined Science: Trilogy) : Revision Note
Specific Latent Heat
A certain amount of energy is required to change the state of a certain mass of a substance
This amount of energy is known as the latent heat
The specific latent heat is defined as:
The amount of energy required to change the state of 1 kg of a substance with no change in temperature
There are two types of specific latent heat:
Specific latent heat of fusion
Changing the state between a solid and liquid
Solid to liquid, or liquid to solid
Specific latent heat of vaporisation
Changing the state between a liquid and gas
Liquid to gas, or gas to liquid
Latent heat is represented by the symbol L with units joules per kilogram (J/kg)
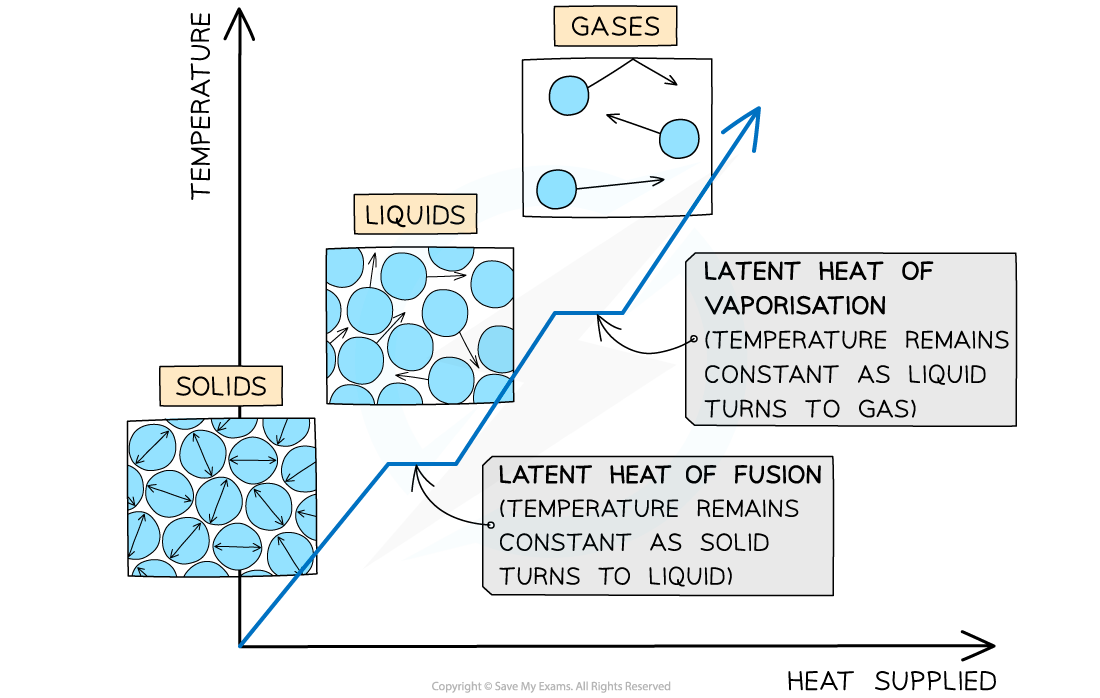
The changes of state with heat supplied against temperature
Latent Heat of Fusion
The specific latent heat of fusion is defined as:
The energy required to convert 1 kg of a substance between a solid and a liquid state with no change in temperature
This applies when melting a solid or freezing a liquid
When a solid substance is melted, its temperature stays constant until all of the substance has melted
The latent heat of fusion is the amount of energy needed per kg for all the particles in the substance to overcome the intermolecular forces of attraction holding them together in their solid state
If a substance in its liquid state is frozen, the substance will solidify at the same temperature as its melting point
In this case, the latent heat of fusion is the amount of energy per kg transferred away from the substance until all the particles in the substance have succumbed to the intermolecular forces of attraction that hold them together in their solid structure
Latent Heat of Vaporisation
The specific latent heat of vaporisation is defined as:
The energy required to convert 1 kg between a liquid and a gaseous state with no change in temperature
This applies when vaporising a liquid or condensing a gas
When a liquid substance is vaporised, its temperature will stay constant until all of the substance has vaporised
The latent heat of vaporisation is the amount of energy per kg needed for all the particles in the substance to overcome the intermolecular forces of attraction holding them together in their liquid state
If a substance is a gas and is condensed, it will condense at the same temperature as its boiling point
In this case, the latent heat of vaporisation is the amount of energy per kg transferred away from the substance until all the particles in the substance have succumbed to the intermolecular forces of attraction that hold them together in their liquid state
Examiner Tips and Tricks
The specific latent heat of fusion and vaporisation value of all substances will be provided for you in the exam question, so you do not need to memorise the value of any.
However, make sure to include 'with no change in temperature' in your definition of specific latent heat to be awarded full marks.
Use these reminders to help you remember which type of latent heat is being referred to:
Latent heat of fusion = imagine ‘fusing’ the liquid molecules together to become a solid
Latent heat of vaporisation = “water vapour” is steam, so imagine vaporising the liquid molecules into a gas
But remember that the change of state can go in either direction!
Latent just means hidden. So the energy being transferred into the system (by heating it) causes the temperature to rise to the melting point of a substance; we can see this happening using a thermometer. But when the substance reaches its melting point, we can continue to transfer energy to the system, but we don’t see the temperature rising any more; it stays at the melting point. So what happens to this energy? What is it being used for? It appears to be hidden or latent because we can’t see its effects.
Once the substance has fully melted, the temperature begins to rise again. And when it reaches the boiling point, the same thing happens. The temperature stays constant even though energy is still being transferred into the system. What do these two events have in common? The period of latent energy transfers happened as the substance was changing state. Therefore, the energy transferred to the system at these points must be used for the state change. The energy is transferred to the molecules or particles and they use it to overcome the intermolecular forces of attraction holding them in their solid or liquid state. Physicists call this latent heat.
Specific Latent Heat Equation
The amount of energy E required to melt or vaporise a mass of m with latent heat L is:
E = mL
Where:
E = thermal energy required for a change in state, in joules (J)
m = mass, in kilograms (kg)
L = specific latent heat, in joules per kilogram (J/kg)
This equation can be rearranged with the help of a formula triangle:

For context, the values of latent heat for water are:
Specific latent heat of fusion = 330 kJ/kg
Specific latent heat of vaporisation = 2.26 MJ/kg
Therefore, evaporating 1 kg of water requires roughly seven times more energy than melting the same amount of ice to form water
Worked Example
Calculate the energy transferred to the surroundings as 0.60 kg of stearic acid changed state from liquid to solid. The specific latent heat of fusion of stearic acid is 199 000 J/kg.
Answer:
Step 1: List the known quantities
Mass, m = 0.60 kg
Specific latent heat of fusion, L = 199 000 J/kg
Step 2: Write down the relevant equation
E = mL
Step 3: Substitute in the values
E = 0.60 × 199 000 = 119 400 J
Examiner Tips and Tricks
Remember that L is used as the symbol of specific latent heat of fusion or vaporisation. This equation will be given on your equation sheet, however, it is important you know how to use it!

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
