Required Practical: Determining Density (AQA GCSE Combined Science: Trilogy): Revision Note
Exam code: 8464
Did this video help you?
Required Practical 5: Determining Density
Equipment List

Resolution of measuring equipment:
30 cm ruler = 1 mm
Vernier calipers = 0.01 mm
Micrometer = 0.001 mm
Digital balance = 0.01 g
Experiment 1: Measuring the Density of Regularly Shaped Objects
The aim of this experiment is to determine the densities of regular objects by using measurements of their dimensions
Variables:
Independent variable = Type of shape / volume
Dependent variable = Mass of the object
Method

Place the object on a digital balance and note down its mass
Use either the ruler, Vernier calipers or micrometer to measure the object’s dimensions (width, height, length, radius) – the apparatus will depend on the size of the object
Repeat these measurements and take an average of these readings before calculating the density
An example of a results table might look like this:

Analysis of Results
Calculate the volume of the object depending on whether it is a cube, sphere, cylinder (or other regular shape)

Calculating the volume of an object depends on its shape
Remember to convert from centimetres (cm) to metres (m) by dividing by 100
1 cm = 0.01 m
50 cm = 0.5 m
Using the mass and volume, the density of each can be calculated using the equation:
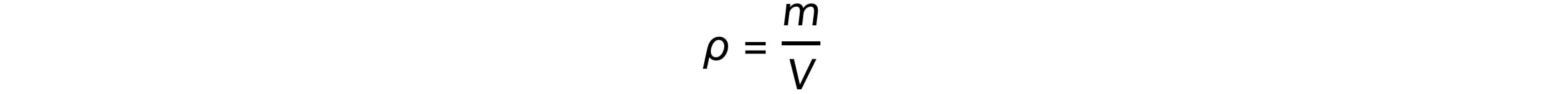
Where:
ρ = density in kilogram per metres cubed (kg/m3)
m = mass in kilograms (kg)
V = volume in metres cubed (m3)
Experiment 2: Measuring the Density of Irregularly Shaped Objects
The aim of this experiment is to determine the densities of irregular objects using a displacement technique
Variables:
Independent variable = Different irregular shapes / mass
Dependent variable = Volume of displaced water
Method

Apparatus for measuring the density of irregular objects
Place the object on a digital balance and note down its mass
Fill the eureka can with water up to a point just below the spout
Place an empty measuring cylinder below its spout
Carefully lower the object into the eureka can
Measure the volume of the displaced water in the measuring cylinder
Repeat these measurements and take an average before calculating the density
An example of a results table might look like this:

Analysis of Results
The volume of the water displaced is equal to the volume of the object
Once the mass and volume of the shape are known, the density can be calculated using:
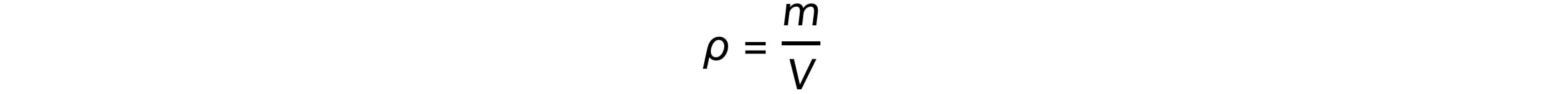
Experiment 3: Measuring Density of Liquids
The aim of this experiment is to determine the density of a liquid by finding a difference in its mass
Variables:
Independent variable = Volume of water added
Dependent variable = Mass of cylinder
Method

Apparatus for determining the density of a liquid
Place an empty measuring cylinder on a digital balance and note down the mass
Fill the cylinder with the liquid and note down the volume
Note down the new reading on the digital balance
Repeat these measurements and take an average before calculating the density
An example of a results table might look like this:

Analysis of Results
Find the mass of the liquid by subtracting the final reading from the original reading
Mass of liquid = Mass of cylinder with water – mass of cylinder
Remember to convert between grams (g) and kilograms (kg) by dividing by 1000
1 g = 0.001 kg
78 g = 0.078 kg
Once the mass and volume of the liquid are known, the density can be calculated using the equation:
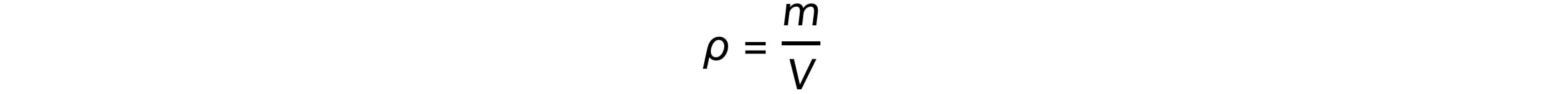
Evaluating the Experiments
Systematic Errors:
Ensure the digital balance is set to zero before taking measurements of mass
This includes when measuring the density of the liquid – remove the measuring cylinder and zero the balance before adding the liquid
Random Errors:
A main cause of error in this experiment is in the measurements of length
Ensure to take repeat readings and calculate an average to keep this error to a minimum
Place the irregular object in the displacement can carefully, as dropping it from a height might cause water to splash which will lead to an incorrect volume reading
Safety Considerations
There is a lot of glassware in this experiment, ensure this is handled carefully
Water should not be poured into the measuring cylinder when it is on the electric balance
This could lead to electric shock
Make sure to stand up during the whole experiment, to react quickly to any spills
Examiner Tips and Tricks
There is a lot of information to take in here! When writing about experiments, a good sequence is as follows:
If you need to use an equation to calculate something, start off by giving it as this will give you some hints about what you need to mention later
List the apparatus that you need
State what measurements you need to make (your equation will give you some hints) and how you will measure them
Finally, state that you will repeat each measurement several times and take averages
The most common point for errors to occur is in the displacement of water from the eureka can to the measuring cylinder.
Make sure the eureka can is completely full before adding the object - you want to make sure that all the displaced water exits the can, if the can is not full, then the water level can rise before it begins to spill out
Make sure that all the displaced water goes into the measuring cylinder - if you spill some then you are not getting an accurate measurement
If you notice one of these errors during the investigation, then stop and redo that step
Remember that anomalous results should not be included in any averages you calculate. It can be easy to spot an anomalous result on a graph, but it is much more difficult to spot one from a group of data. So if you notice one during your investigation, you can correct for it by rerunning that part of the investigation.

Unlock more, it's free!
Did this page help you?