Nuclear Equations for Fission & Fusion (WJEC GCSE Physics): Revision Note
Exam code: 3420
Balancing Nuclear Equations for Fission & Fusion
Nuclear Fission Equations
An example of a fission reaction is uranium-235 being bombarded with a neutron to produce two daughter nuclei, three neutrons and energy in the form of gamma radiation
This can be written in the form of an equation:
+ energy
Where:
is an isotope of uranium
is a neutron
is an isotope of krypton (one daughter nucleus)
is an isotope of barium (the other daughter nucleus)
The sum of the top (nucleon) numbers on the left-hand side equals the sum of the top number on the right-hand side: 235 + 1 = 92 + 141 + (3 × 1)
The same is true for the lower (proton) numbers: 92 + 0 = 36 + 56 + (3 × 0)
Representing Nuclear Fission Reactions
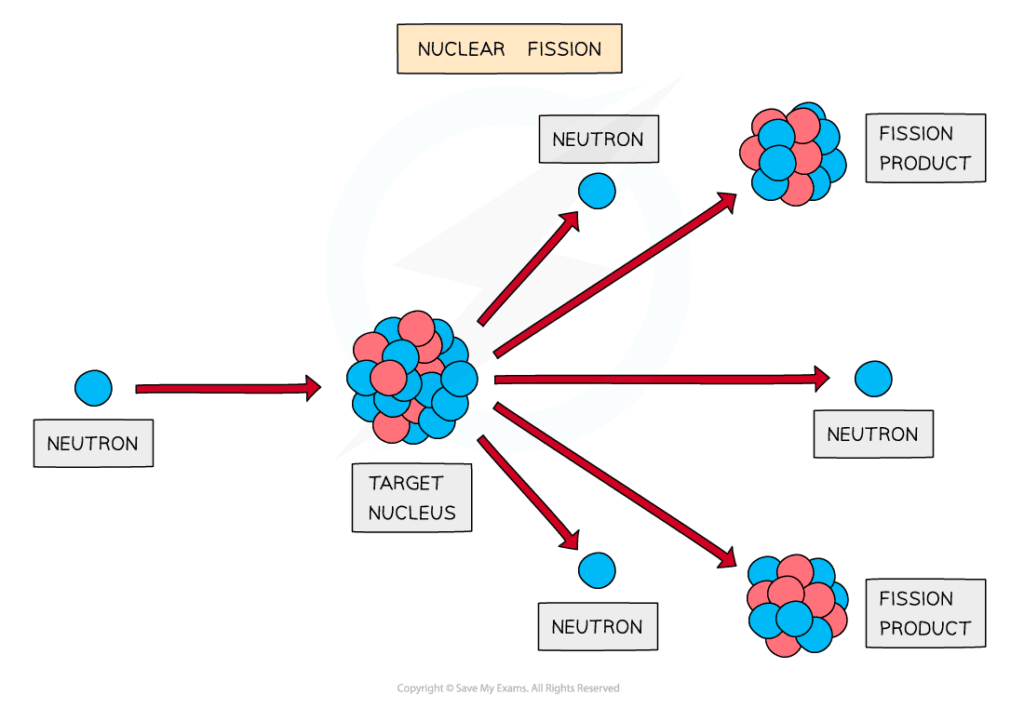
A neutron is fired at a target nucleus, causing it to split into two fission products plus 3 neutrons
The above equation represents a fission reaction where
A uranium nucleus absorbs a neutron
It splits into two smaller nuclei – a krypton nucleus and a barium nucleus
Three neutrons are released in the process, along with a large amount of energy
Nuclear Fusion Equations
An example of a fusion reaction is deuterium and tritium nuclei combining at high energies to produce a helium nucleus, a neutron and a lot of energy
This can be written as a nuclear equation:
+ energy
Where:
is deuterium (an isotope of hydrogen with 1 proton and 1 neutron)
is tritium (an isotope of hydrogen with 1 proton and 2 neutrons)
is a stable helium nucleus
is a neutron
The sum of the top (nucleon) numbers on the left-hand side equals the sum of the top number on the right-hand side: 2 + 3 = 4 + 1
The same is true for the lower (proton) numbers: 1 + 1 = 2 + 0
Representing Nuclear Fusion Reactions
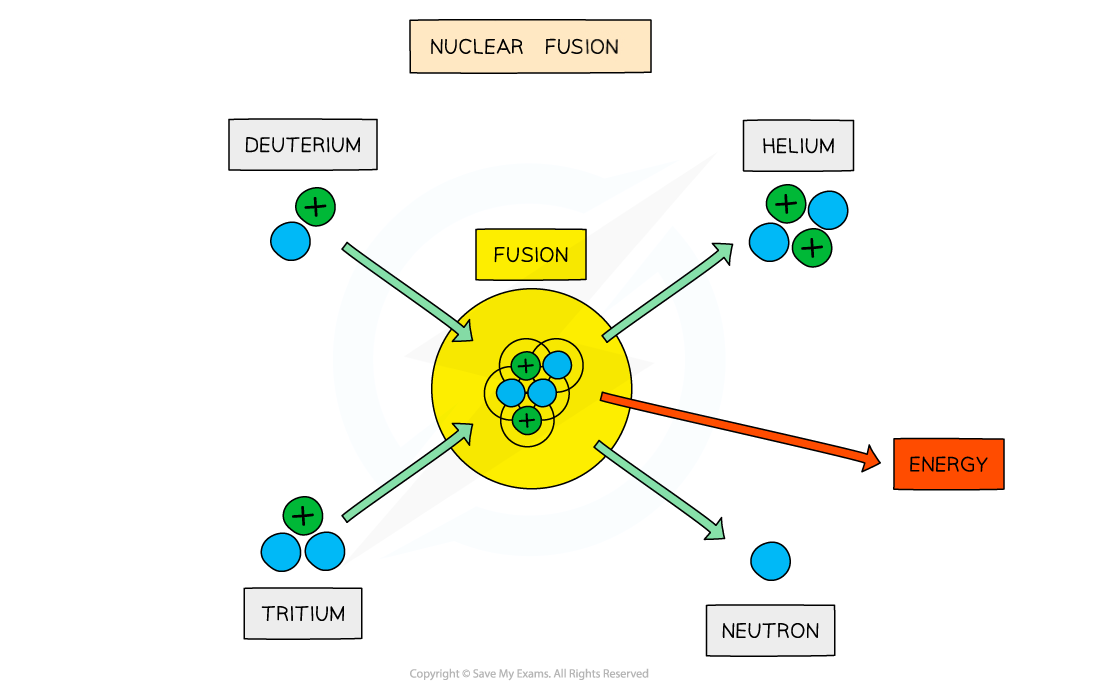
Isotopes of hydrogen, deuterium and tritium, can fuse into a helium nucleus and a neutron plus the release of energy
The above equation represents a fusion reaction where
A deuterium nucleus collides with a tritium nucleus
They become close enough to fuse into a helium-4 nucleus
A neutron is released in the process, along with a large amount of energy
Worked Example
A possible nuclear equation for the fission of uranium-235 is
Calculate the number of neutrons N released in this reaction.
Answer:
Step 1: Calculate the nucleon number on the left side of the equation
235 + 1 = 236
Step 2: Calculate the nucleon number on the right side of the equation
96 + 138 + (N × 1) = 233 + N
Step 3: Equate the nucleon number for both sides of the equation
236 = 233 + N
Step 4: Rearrange for the number of neutrons, N
N = 236 – 233 = 3
Worked Example
One of the nuclear fusion reactions that occurs in the Sun is shown below.
Complete the equation.
Answer:
Step 1: Recall the proton number for hydrogen and the meaning of isotope
Isotopes are elements with the same proton number but different nucleon numbers
Hydrogen has a proton number of 1
So, the bottom number for both hydrogen nuclei is 1
Step 2: Determine the nucleon numbers of the hydrogen isotopes
The total nucleon number on the right side of the equation is 3, as gamma is an electromagnetic wave so does not have a nucleon or proton number
The hydrogen nuclei on the left side are isotopes
Therefore, their nucleon numbers must be 1 and 2
Step 3: Write the equation out in full and check both sides
Nucleon numbers: 1 + 2 = 3 + 0
Proton numbers: 1 + 1 = 2 + 0
The numbers balance, so the equation must be correct
Writing Nuclear Equations for Fission & Fusion
Higher Tier Only
Given some information about different nuclei, you could be asked to write full, balanced nuclear equations for particular nuclear reactions, such as fission or fusion
Remember the key rule for writing balanced equations:
The sum of the nucleon numbers and proton numbers must be equal before and after the reaction
In nuclear fusion equations:
Two small nuclei react
One larger nucleus is produced
There may be a neutron released, but not always
In nuclear fission equations:
One large nucleus absorbs a neutron
Two smaller nuclei are produced
Two or three neutrons are always released
The worked examples below illustrate the kinds of questions that could be asked
Worked Example
The diagram below shows an example of a nuclear fusion reaction in which two isotopes of helium fuse together to make a beryllium-7
nucleus plus the emission of a gamma ray.
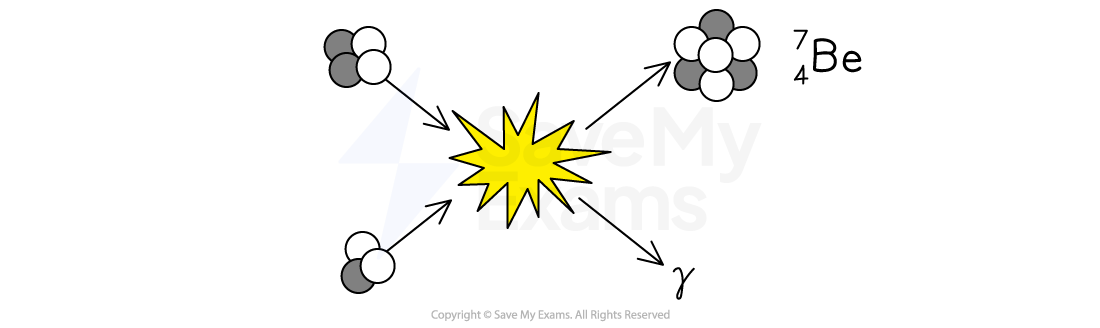
Write a balanced nuclear equation for this fusion reaction.
Answer:
Step 1: Write a word equation using the information in the question
Underline or highlight the keywords in the question:
"Two isotopes of helium fuse together to make a beryllium-7 nucleus plus the emission of a gamma ray"
Write a word equation for the reaction:
helium + helium → beryllium-7 + gamma
Step 2: Work out the symbols for the helium isotopes
The
notation for beryllium-7 is
This means it contains 4 protons and 3 neutrons
Using the diagram, we can deduce the light particles are protons and the dark particles are neutrons
Isotopes are elements with the same proton number but different nucleon numbers
So, both helium nuclei must have a proton number of 2
The two helium isotopes are therefore:
Helium-3:
(2 protons, 1 neutron)
Helium-4:
(2 protons, 2 neutrons)
Gamma particles have no mass or charge so have nucleon and proton numbers of 0
Step 3: Write the balanced nuclear equation using the correct symbols
Nucleon numbers: 3 + 4 = 7 + 0
Proton numbers: 2 + 2 = 4 + 0
The numbers balance, so the equation must be correct
Worked Example
The table shows some information about different nuclei involved in a nuclear fission reaction.
Nucleus | Symbol | Number of protons | Number of neutrons |
Uranium | U | 92 | 143 |
Barium | Ba | 56 | 88 |
Krypton | Kr | 36 | 53 |
A nucleus of uranium undergoes fission when bombarded with a slow-moving neutron (n) causing it to split into the daughter nuclei barium and krypton plus the release of further neutrons.
Write a balanced nuclear equation for this fission reaction.
Answer:
Step 1: Write a word equation using the information in the question
Underline or highlight the keywords in the question which tell us about the nuclei on the left-hand side (LHS):
"A nucleus of uranium undergoes fission when bombarded with a slow-moving neutron"
Underline or highlight the keywords in the question which tell us about the nuclei on the right-hand side (RHS):
"causing it to split into the daughter nuclei barium and krypton plus the release of further neutrons"
Write a word equation for the reaction:
uranium + neutron → barium + krypton + neutrons
Step 2: Determine the correct symbols for each nucleus or particle
We need to write each nucleus or particle in
notation
It can be useful to draw an extra row and column onto the table to do this (green indicates the changes made to the table):
Nucleus | Symbol | Number of protons Z | Number of neutrons | Nucleon number A |
Uranium | 92 | 143 | 92 + 143 = 235 | |
Barium | 56 | 88 | 56 + 88 = 144 | |
Krypton | 36 | 53 | 36 + 53 = 89 | |
Neutron | 0 | 1 | 0 + 1 = 1 |
Step 3: Write the nuclear equation using the symbols
Step 4: Balance the equation by checking the total nucleon and proton numbers on each side
Total proton number:
On the LHS: 92 + 0 = 92
On the RHS: 56 + 36 + 0 = 92
The total proton number is the same on both sides
Total nucleon number:
On the LHS: 235 + 1 = 236
On the RHS: 144 + 89 + 1 = 234
There is a difference of 2, and the question states there is a "release of further neutrons"
This means there must be 2 extra neutrons released i.e. 3 neutrons in total
To represent this, we put a 3 in front of the neutron symbol on the RHS, so, the fully balanced equation is:

Unlock more, it's free!
Did this page help you?