Heat Transfer & Changes in Temperature (WJEC GCSE Physics): Revision Note
Exam code: 3420
Heat Transfer & Changes in Temperature
If the temperature of the system increases, the increase in temperature of this system depends on:
The mass of the substance heated
The type of material
The energy input to the system
Thermal energy is transferred into the system when a substance is heated
The amount of energy needed to raise the temperature of a given mass by a given amount can be calculated using the equation:
Q = mcΔθ
Where:
Q = change in energy, in joules (J)
m = mass, in kilograms (kg)
c = specific heat capacity, in joules per kilogram per degree Celsius (J/kg °C)
Δθ = change in temperature, in degrees Celsius (°C)
The specific heat capacity, c of a substance is defined as:
The amount of energy required to raise the temperature of 1 kg of the substance by 1 °C
Different substances have different specific heat capacities
If a substance has a low specific heat capacity, it heats up and cools down quickly
It takes less energy to change its temperature
If a substance has a high specific heat capacity, it heats up and cools down slowly
It takes more energy to change its temperature
Specific Heat Capacity of Different Substances
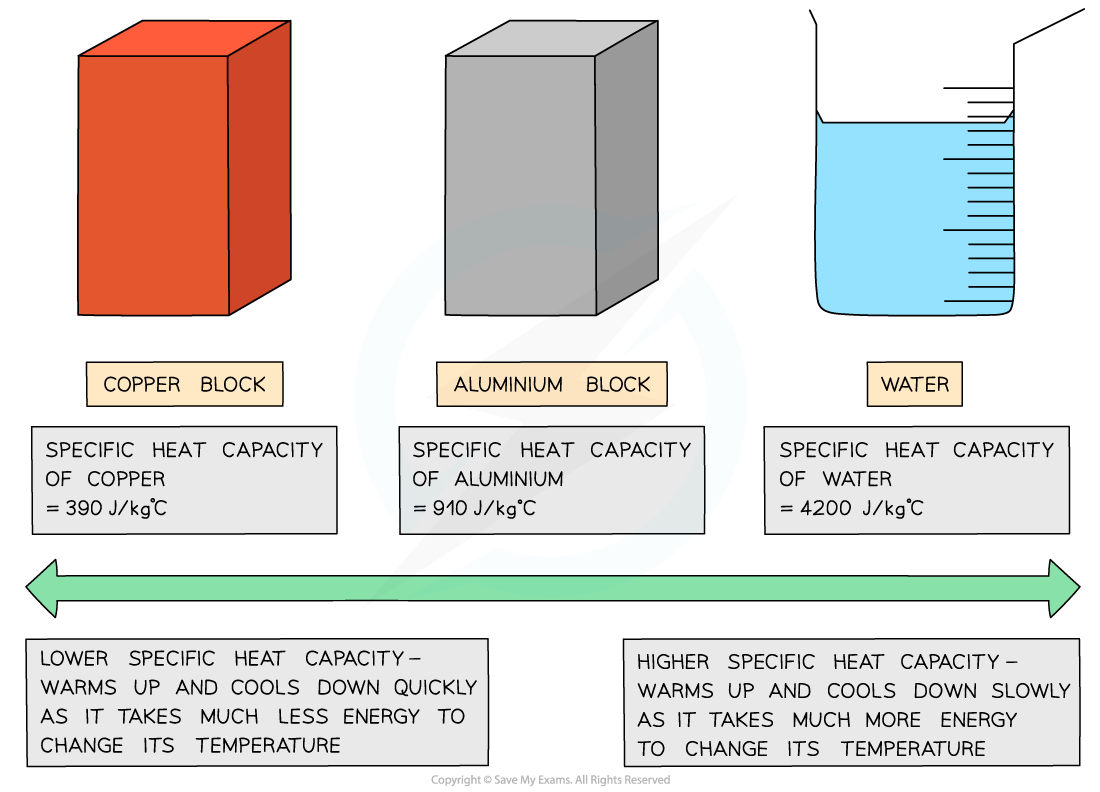
Substances with a low specific heat capacity heat up and cool down quickly, substances with a high specific heat capacity heat up and cool down slowly
Specific heat capacity is mainly used for liquids and solids
The specific heat capacity of different substances determines how useful they would be for a specific purpose
Good electrical conductors, such as copper and lead, are excellent conductors of heat due to their low specific heat capacity
On the other hand, water has a very high specific heat capacity, making it ideal for heating homes as the water remains hot in a radiator for a long time
Worked Example
0.48 kg of water is heated in a pan. The temperature of the water is increased by 0.70 °C.
The specific heat capacity of water is 4200 J / kg °C.
Calculate the amount of energy transferred to the water.
Answer:
Step 1: List the known quantities
Mass of water, m = 0.48 kg
Change in temperature, Δθ = 0.70 °C
Specific heat capacity of water, c = 4200 J / kg °C
Step 2: Write out the equation
Step 3: Substitute in the known values to calculate
Round to 2 significant figures
Examiner Tips and Tricks
You will always be given the specific heat capacity of a substance, so you do not need to memorise any of the values mentioned here.
You should give your answer to the same number of significant figures as the least precise value of your input data.

Unlock more, it's free!
Did this page help you?