Heat Transfer & Changes in State (WJEC GCSE Physics): Revision Note
Exam code: 3420
Heat Transfer & Changes in State
A certain amount of energy is required to change the state of a certain mass of a substance
This amount of energy is known as the latent heat
The specific latent heat is defined as:
The amount of energy required to change the state of 1 kg of a substance with no change in temperature
Latent heat can be calculated using:
Q = mL
Where:
Q = thermal energy required for a change in state, in joules (J)
m = mass, in kilograms (kg)
L = specific latent heat, in joules per kilogram (J/kg)
Higher Tier students will need to be able to rearrange this equation
Thermal Energy, Mass and Specific Latent Heat Equation Triangle

Cover up the variable you need to calculate, and the triangle shows you how the equation is arranged
Changing a liquid to a gas requires more energy than changing a solid to a liquid
The energy required to change the state of a substance between a solid and a liquid is called the latent heat of fusion
This includes:
A solid melting into a liquid
A liquid freezing into a solid
The energy required to change the state of a substance between a liquid and a gas is called the latent heat of vaporisation
This includes:
A liquid boiling into a gas
A gas condensing into a liquid
For example the latent heat of fusion for water is 336 kJ, whereas the latent heat of vaporisation is 2260 kJ
Worked Example
Calculate the energy transferred to the surroundings as 0.60 kg of stearic acid changes state from liquid to solid.
The specific latent heat of fusion of stearic acid is 199 000 J / kg.
Answer:
Step 1: List the known quantities
Mass, m = 0.60 kg
Specific latent heat of fusion, L = 199 000 J / kg
Step 2: Write down the relevant equation
Q = mL
Step 3: Substitute in the values
Q = 0.60 × 199 000
Q = 119 400 J
Examiner Tips and Tricks
Remember that L is used as the symbol of specific latent heat of fusion or vaporisation, it is just the values that will be different.
Explaining Changes in Temperature & State
Higher Tier Only
Latent Heat of Fusion
The specific latent heat of fusion is defined as:
The energy required to convert 1 kg of a substance between a solid and a liquid state with no change in temperature
This applies when melting a solid or freezing a liquid
Melting and freezing both occur at the melting point of the substance
When a solid substance is melting, its temperature stays constant until all of the substance has melted
The latent heat of fusion is the amount of energy needed per kg for all the particles in the substance to break the bonds holding them together in their solid state
When a solid substance is freezing, its temperature stays constant until all of the substance has frozen
The latent heat of fusion is the amount of energy released per kg when all the particles in the substance form the bonds that hold them together in their solid state
Latent Heat of Vaporisation
The specific latent heat of vaporisation is defined as:
The energy required to convert 1 kg between a liquid and a gaseous state with no change in temperature
This applies when vaporising a liquid or condensing a gas
Vaporisation and condensing both occur at the boiling point of the substance
When a liquid is boiling, its temperature stays constant until all of the substance has vapourised
The latent heat of vaporisation is the amount of energy needed per kg for all the particles in the substance to break the bonds holding them together in their liquid state
When a liquid is condensing, its temperature stays constant until all of the substance has condensed
The latent heat of vaporisation is the amount of energy released per kg when all the particles in the substance form the bonds that hold them together in their liquid state
Graph of Phase Changes for a Substance
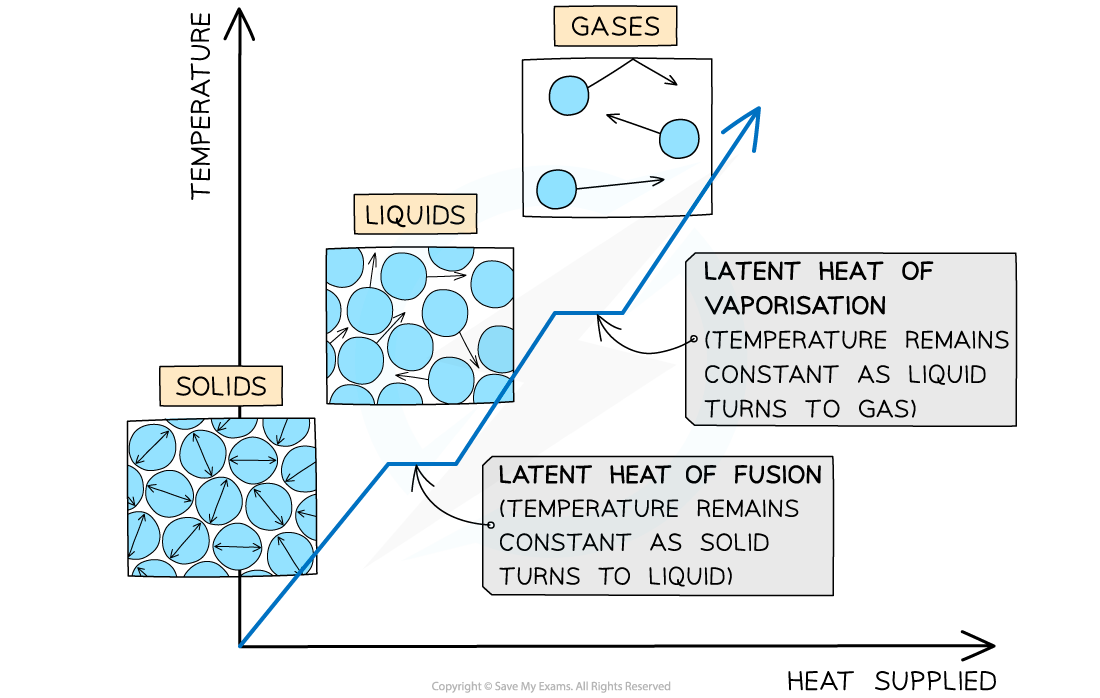
The flat areas of the graph show the phase change
Examiner Tips and Tricks
The specific latent heat of fusion and vaporisation value of all substances will be provided for you in the exam question, so you do not need to memorise the value of any.
However, make sure to include 'with no change in temperature' in your definition of specific latent heat to be awarded full marks.
Use these reminders to help you remember which type of latent heat is being referred to:
Latent heat of fusion = imagine ‘fusing’ the liquid molecules together to become a solid
Latent heat of vaporisation = “water vapour” is steam, so imagine vaporising the liquid molecules into a gas
But remember that the change of state can go in either direction!

Unlock more, it's free!
Did this page help you?