Gas Laws (WJEC GCSE Physics): Revision Note
Exam code: 3420
Behaviour of Gases with Changing Conditions
Pressure & Volume
For a gas at constant temperature:
Pressure increases if the volume decreases
Pressure decreases if the volume increases
Pressure and volume are inversely proportional
Graph of Relationship Between Volume and Pressure
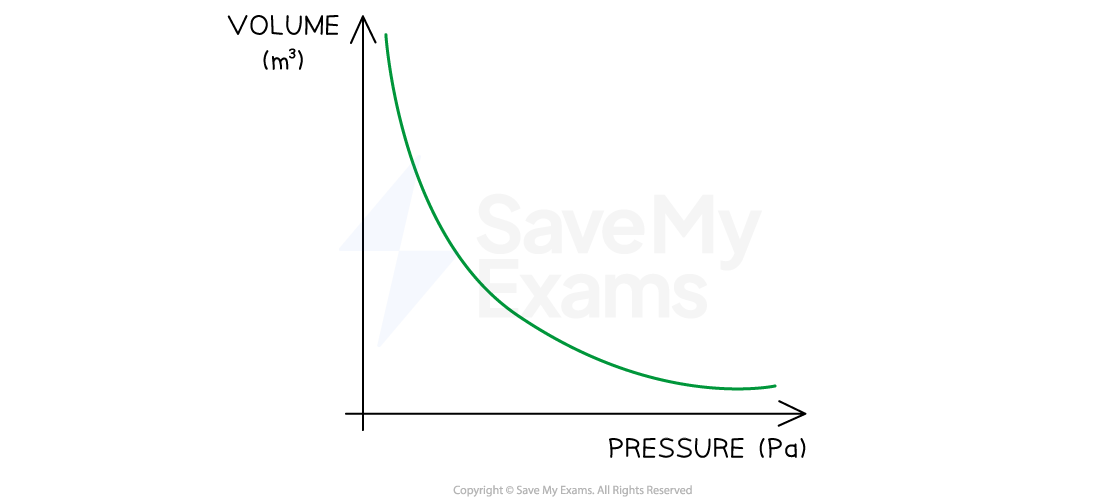
As pressure increases, volume decreases and vice versa. The curved shape of the graph shows an inversely proportional relationship
Pressure & Temperature
For a gas at constant volume:
The pressure increases if the temperature increases
The pressure decreases if the temperature decreases
Pressure and temperature are directly proportional
Graph of Relationship Between Pressure and Temperature on the Kelvin Scale
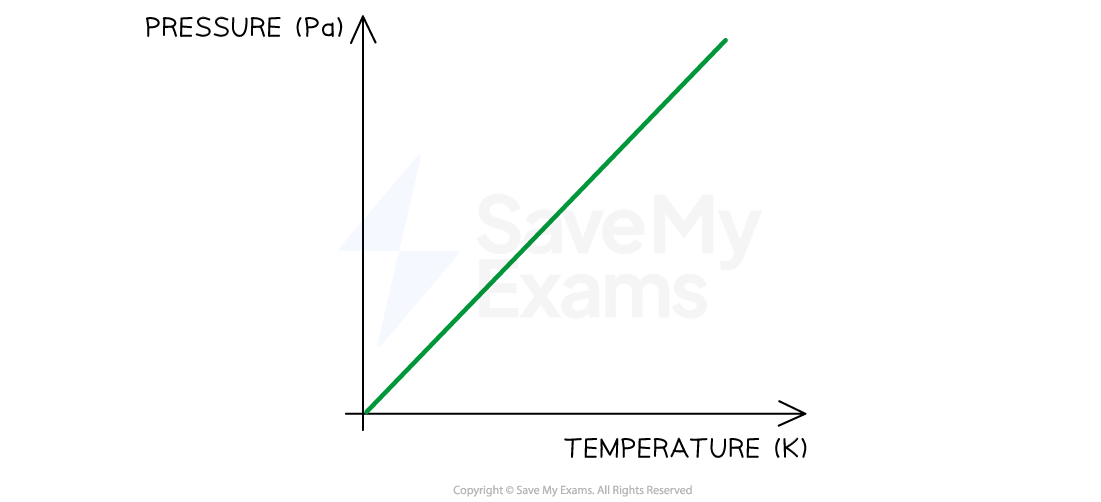
As temperature increases, pressure increases. The shape of the graph shows a directly proportional relationship
Volume and Temperature
For a gas at constant pressure:
The volume will increase if the temperature is increased
The volume will decrease if the temperature is decreased
Volume and temperature are directly proportional
Graph of Relationship Between Volume and Temperature on the Kelvin Scale
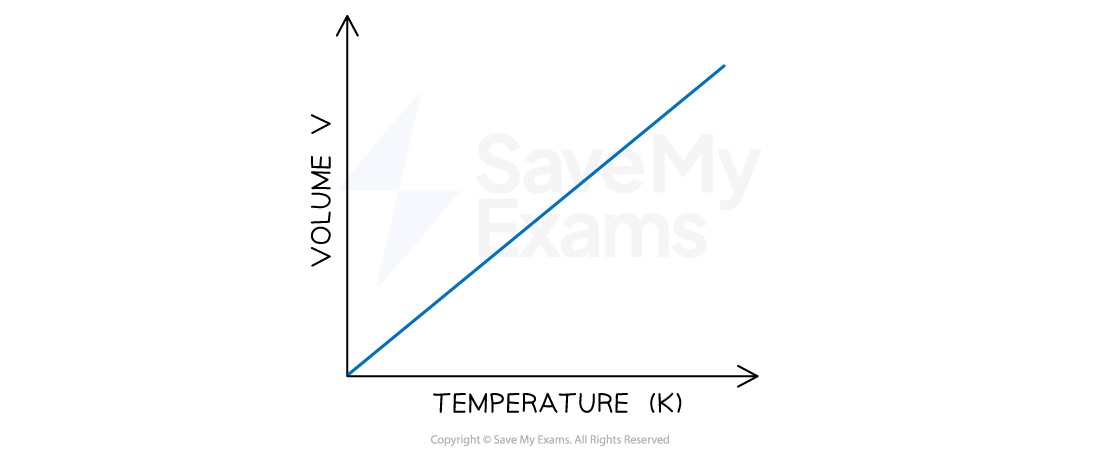
As temperature increases, volume increases. The shape of the graph shows a directly proportional relationship
Explaining Changes in Pressure
Changes in Pressure With Changing Volume
In a gas, the particles are spread out with spaces between them
This makes a gas easy to expand and compress
When a gas is compressed, the volume is decreased
The density of the gas increases
The volume of the container has decreased but the number of particles has remained the same
This results in more frequent collisions with the container walls
So there is an increase in pressure
When a gas is expanded, the volume is increased
The density of the gas decreases
The volume of the container has increased but the number of particles has remained the same
This results in less frequent collisions with the container walls
So there is a decrease in pressure
Compression of a Gas at Constant Volume
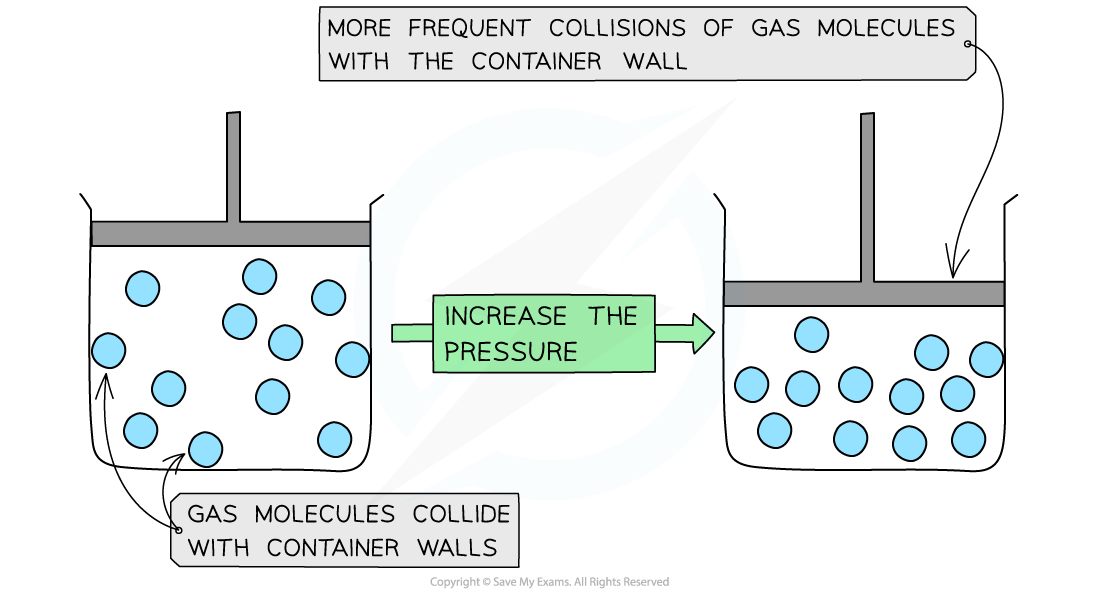
As the volume of the container is decreased, the gas particles collide more frequently with the walls of the container causing an increase in pressure
The key assumption is that the temperature and the mass (and number) of the particles remain the same
Pressure Changes with Changing Temperature
The temperature of a substance is related to the average kinetic energy of its particles
As a substance is heated, the particles gain more kinetic energy and move around faster
When the temperature of a gas is increased, the pressure is increased
The particles of the gas gain more kinetic energy and move around faster
This results in more frequent collisions with the container walls
And the collisions have a greater force
This results in an increase in pressure
When the temperature a gas is decreased, the pressure is decreased
The particles of the gas have less kinetic energy and move around slower
This results in less frequent collisions with the container walls
And the collisions have a lesser force
This results in a decrease in pressure
Heating a Gas at Constant Volume
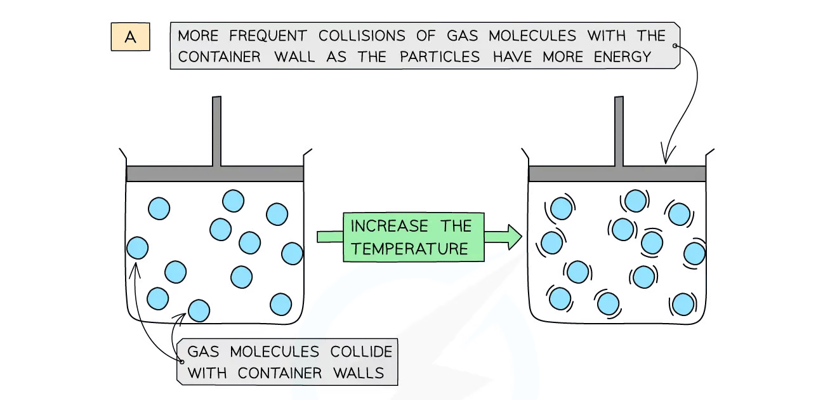
As the temperature of the gas is increased, the gas particles collide more frequently and more forcefully with the walls of the container, causing an increase in pressure
The pressure−temperature graph (and the volume−temperature graph) look a little different on the Celsius scale
The line only goes through the origin if the temperature is in K
On the Celsius scale, zero pressure is reached at absolute zero, −273 °C
Pressure-Temperature Graph on the Celsius Scale

The line does not go through the origin when the temperature is given in °C, zero pressure is reached at −273 °C
Gas Laws
Higher Tier Only
For a fixed mass of a gas held at a constant temperature:
Pressure is inversely proportional to volume
Therefore:
Where:
p = pressure in pascals (Pa)
V = volume in metres cubed (m3)
Diagram Showing a Decrease in Pressure When Volume is Increased
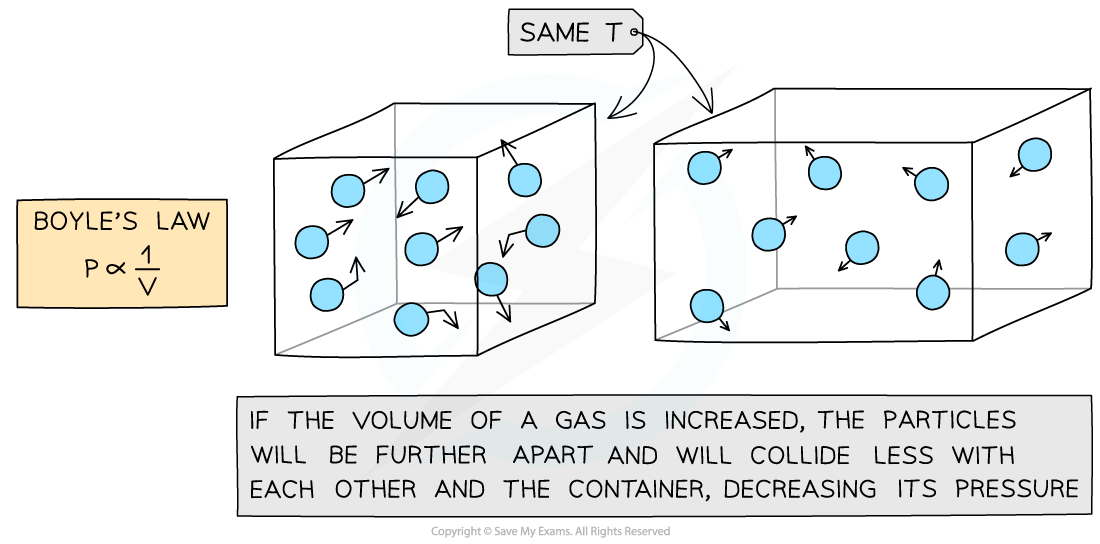
Increasing the volume of a gas decreases its pressure
The
equation can also be written as:
Where:
p1 = initial pressure in pascals (Pa)
V1 = initial volume in metres cubed (m3)
p2 = final pressure in pascals (Pa)
V2 = final volume in metres cubed (m3)
This equation is sometimes referred to as Boyle's Law
It is used to compare the pressure and volume before and after a change in a gas
Diagram Illustrating Boyle's Law
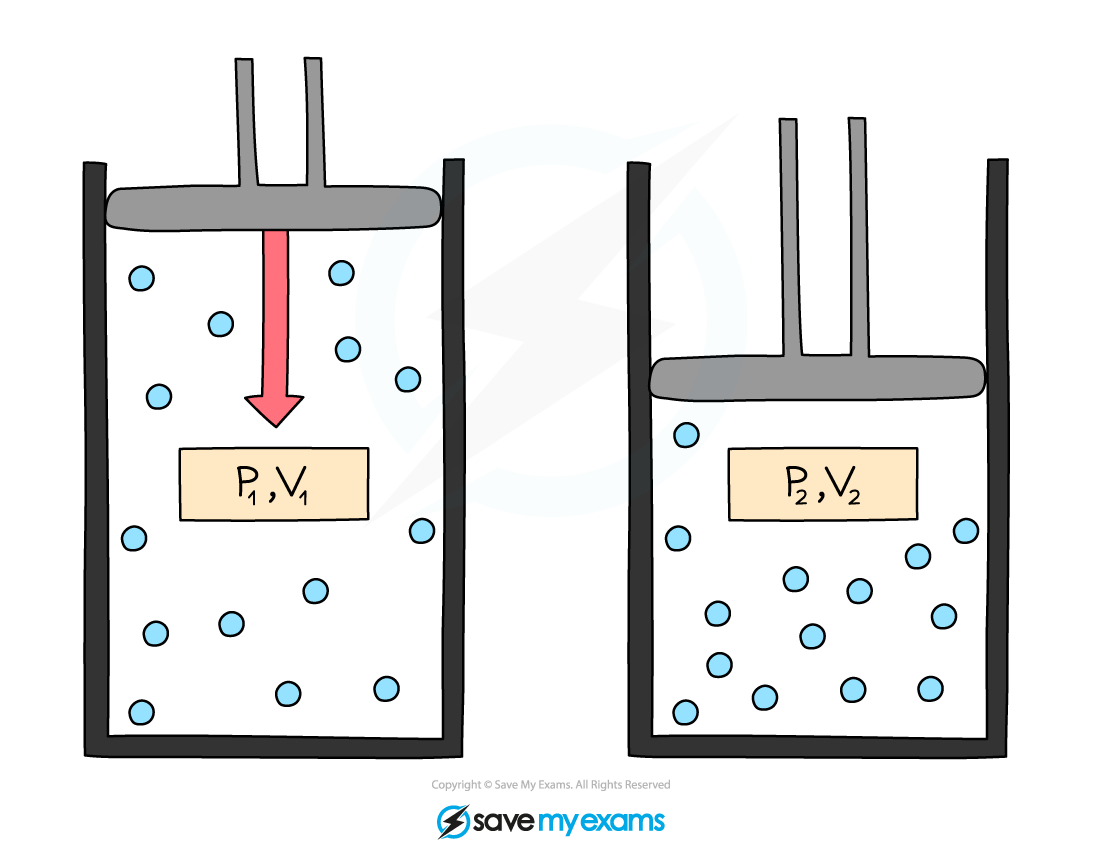
The initial pressure and volume are the values before the change, and the final pressure and volume are the values after the change
Worked Example
A gas occupies a volume of 0.70 m3 at a pressure of 200 Pa.
Calculate the pressure exerted by the gas after it has been compressed to a volume of 0.15 m3.
Assume that the temperature and mass of the gas stay the same.
Answer:
Step 1: List the known variables
Initial pressure, p1 = 200 Pa
Initial volume, V1 = 0.70 m3
Final volume, V2 = 0.15 m3
Step 2: Write out the equation
Step 3: Rearrange the equation to make p2 the subject
Divide both sides by V2
Step 4: Substitute in the known values to calculate
Round to 2 significant figures
Examiner Tips and Tricks
Always check whether your final answer makes sense. If the gas has been compressed, the final pressure is expected to be more than the initial pressure (like in the worked example). If this is not the case, double-check the rearranging of any formulae and the values put into your calculator. One pascal is a very small amount of pressure, and you will typically meet pressures in the order of kilo-pascals. The pressure on you at the moment because of the air around you is equal to 100 kPa, so use this as a reference when considering if your answer makes sense.
Foundation Tier students would be given the equation in the exam question in its rearranged form.

Unlock more, it's free!
Did this page help you?