Nuclear Fission (OCR GCSE Physics A (Gateway)) : Revision Note
Nuclear Fission
There is a lot of energy stored within the nucleus of an atom
This energy can be released in a nuclear reaction such as fission or fusion
Nuclear fission is defined as:
The splitting of a large, unstable nucleus into two smaller nuclei
Isotopes of uranium and plutonium both undergo fission and are used as fuels in nuclear power stations
During fission, when a neutron collides with an unstable nucleus, the nucleus splits into two smaller nuclei (called daughter nuclei) as well as two or three neutrons
Gamma rays are also emitted

Large nuclei can decay by fission to produce smaller nuclei and neutrons with a lot of kinetic energy
The products of fission move away very quickly
Energy transferred is from nuclear potential energy to kinetic energy
Chain Reactions
It is rare for nuclei to undergo fission without additional energy being put into the nucleus
When nuclear fission occurs in this way it is called spontaneous fission
Usually, for fission to occur the unstable nucleus must first absorb a neutron
Take, for example, uranium-235, which is commonly used as a fuel in nuclear reactors
It has a very long half-life of 700 million years
This means that it would have a low activity and energy would be released very slowly
This is unsuitable for producing energy in a nuclear power station
During induced fission, a neutron is absorbed by the uranium-235 nucleus to make uranium-236
This is very unstable and splits by nuclear fission almost immediately
During the fission, it produces two or three neutrons which move away at high speed
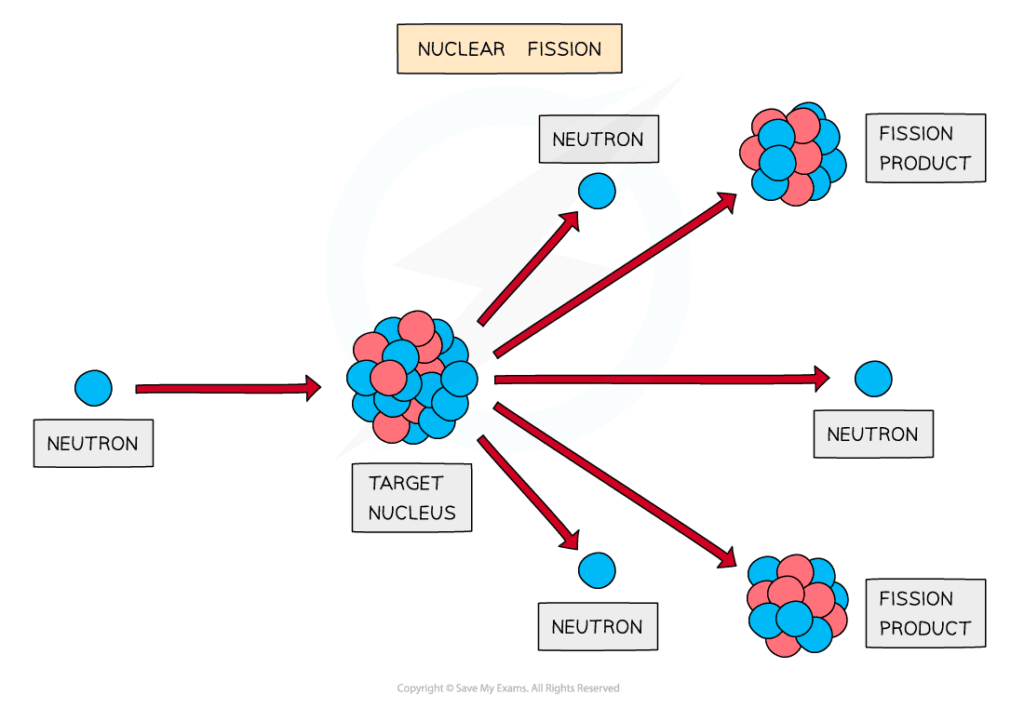
The fission of a nucleus, such as uranium, to produce smaller daughter nuclei with the release of energy
Each of these new neutrons can start another fission reaction, which again creates further excess neutrons
This process is called a chain reaction

The neutrons released by each fission reaction can go on to create further fissions, like a chain that is linked several times – from each chain comes two more
In a nuclear reactor, a chain reaction is required to keep the reactor running
When the reactor is producing energy at the correct rate, the number of free neutrons in the reactor needs to be kept constant
This means some must be removed from the reactor
To do this, nuclear reactors contain control rods
These absorb neutrons without becoming dangerously unstable themselves
Uncontrolled Chain Reactions
Because each new fission reaction releases energy, uncontrolled chain reactions can be dangerous
The number of neutrons available increases quickly, so the number of reactions does too
A nuclear weapon uses an uncontrolled chain reaction to release a huge amount of energy in a short period of time as an explosion
Worked Example
During a particular spontaneous fission reaction, plutonium-239 splits as shown in the equation below:

What is in the missing section of this equation?
Answer:
Step 1: Identify the different mass and atomic numbers
Pu (Plutonium) has mass number 239 and atomic number 94
Pd (Palladium) has mass number 112 and atomic number 46
Cd (Cadmium) has mass number 124 and atomic number 48
Step 2: Calculate the mass and atomic number of the missing section
Mass number is equal to the difference between the mass numbers of the reactants and the products
239 – (112 + 124) = 3
Atomic number is equal to the difference between the atomic numbers of the reactants and the products
94 – (46 + 48) = 0
Step 3: Determine the correct notation
Neutrons have a mass number of 1
Therefore, the missing section must be 3 neutrons
This is written as
Examiner Tips and Tricks
You need to remember that uranium and plutonium are possible elements for fission, but you do not need to know the specific daughter nuclei that are formed.
Use your knowledge of balancing nuclear equations to work these out.

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
