Radioactive Decay (OCR GCSE Physics A (Gateway)) : Revision Note
Radioactive Decay
Unstable Nuclei
Some atomic nuclei are unstable
This is because of an imbalance in the forces within the nucleus
Forces exist between the particles in the nucleus
Carbon-14 is an isotope of carbon which is unstable
It has two extra neutrons compared to stable carbon-12

Carbon-12 is stable, whereas carbon-14 is unstable. This is because carbon-14 has two extra neutrons
Some isotopes are unstable because of their large size or because they have too many or too few neutrons
Radiation
Unstable nuclei can emit radiation to become more stable
Radiation can be in the form of a high energy particle or wave

Unstable nuclei decay by emitting high energy particles or waves
As the radiation moves away from the nucleus, it takes some energy with it
This reduces the overall energy of the nucleus
This makes the nucleus more stable
The process of emitting radiation is called radioactive decay
Radioactive decay is a random process
This means it is not possible to know exactly when a particular nucleus will decay
Types of Radioactive Decay
When an unstable nucleus decays it emits radiation, called nuclear radiation
There are different types of radiation that can be emitted:
Alpha
Beta
Gamma
Neutrons
Alpha Particles
The symbol for alpha is α
An alpha particle is the same as a helium nucleus
This is because they consist of two neutrons and two protons
Alpha particles have a charge of +2
This means they can be affected by an electric field
Beta Particles
The symbol for beta is β
Beta particles are fast-moving electrons
They are produced in nuclei when a neutron changes into a proton and an electron
Beta particles have a charge of -1
This means they can be affected by an electric field
Gamma Rays
The symbol for gamma is γ
Gamma rays are electromagnetic waves
They have the highest energy of the different types of electromagnetic waves
Gamma rays have no charge
Neutrons
The symbol for a neutron is n
Neutrons are one of the two particles found in the nucleus of atoms
Neutrons are neutral, they have no charge

Alpha particles, beta particles, gamma waves and neutrons can be emitted from unstable nuclei
Worked Example
Which of the following statements is not true?
A Isotopes can be unstable because they have too many or too few neutrons
B The process of emitting particles or waves of energy from an unstable nucleus is called radioactive decay
C Scientists can predict when a nucleus will decay
D Radiation refers to the particles or waves emitted from a decaying nucleus
Answer: C
Answer A is true. The number of neutrons in a nucleus determines the stability
Answer B is true. This is a suitable description of radioactive decay
Answer D is true. Radiation is about emissions. It is different to radioactive particles
Answer C is not true
Radioactive decay is a random process
It is not possible to predict precisely when a particular nucleus will decay
Activity
Objects containing radioactive nuclei are called sources of radiation
Sources of radiation decay at different rates which are defined by their activity
The activity is defined as
The rate at which the unstable nuclei from a source of radiation decays
Activity is measured in Becquerels
The symbol for Becquerels is Bq
1 Becquerel is equal to 1 nucleus in the source decaying in 1 second
Worked Example
A source of radiation has an activity of 2000 Bq.
How many unstable atoms decay in 2 minutes?
Answer:
Step 1: Determine the activity
The activity of the source is 2000 Bq
This means 2000 nuclei decay every second
Step 2: Determine the time period in seconds
The time period is 2 minutes
Each minute has 60 seconds
The time period in seconds is:
2 × 60 = 120 seconds
Step 3: Multiply the activity by the time period
Activity (Bq) × Time period (s) = 2000 × 120 = 240 000
Therefore, 240 000 unstable nuclei decay in 2 minutes
Detecting Radiation
Radiation that is emitted from an unstable nucleus can be detected in different ways
For example, photographic film changes colour when exposed to radiation
A Geiger-Muller tube is a device used to detect radiation

This Geiger-Muller Tube is connected to a Geiger Counter. This a common way of detecting radiation and measuring a count-rate
Within the Geiger-Muller tube, ions are created by radiation passing through it
The Geiger-Muller tube can be connected to a Geiger counter
This counts the ions created in the Geiger-Muller tube
Count-rate is the number of decays recorded each second by a detector
Worked Example
A Geiger-Muller tube is used to detect radiation in a particular location. If it counts 16,000 decays in 1 hour, what is the count rate in seconds?
Answer:
Step 1: Identify the different variables
The number of decays is 16 000
The time is 1 hour
Step 2: Determine the time period in seconds
1 hour is equal to 60 minutes, and 1 minute is equal to 60 seconds
Time period = 1 × 60 × 60 = 3600 seconds
Step 3: Divide the total counts by the time period in seconds
Examiner Tips and Tricks
The terms unstable, random and decay have very particular meanings in this topic. Remember to use them correctly when answering questions!
Do not confuse activity and count rate. Activity is the rate at which unstable nuclei decay, whereas count rate is the rate at which radioactive emissions are detected.
Alpha, Beta & Gamma Decay
Alpha Decay
During alpha decay an alpha particle is emitted from an unstable nucleus
A completely new element is formed in the process
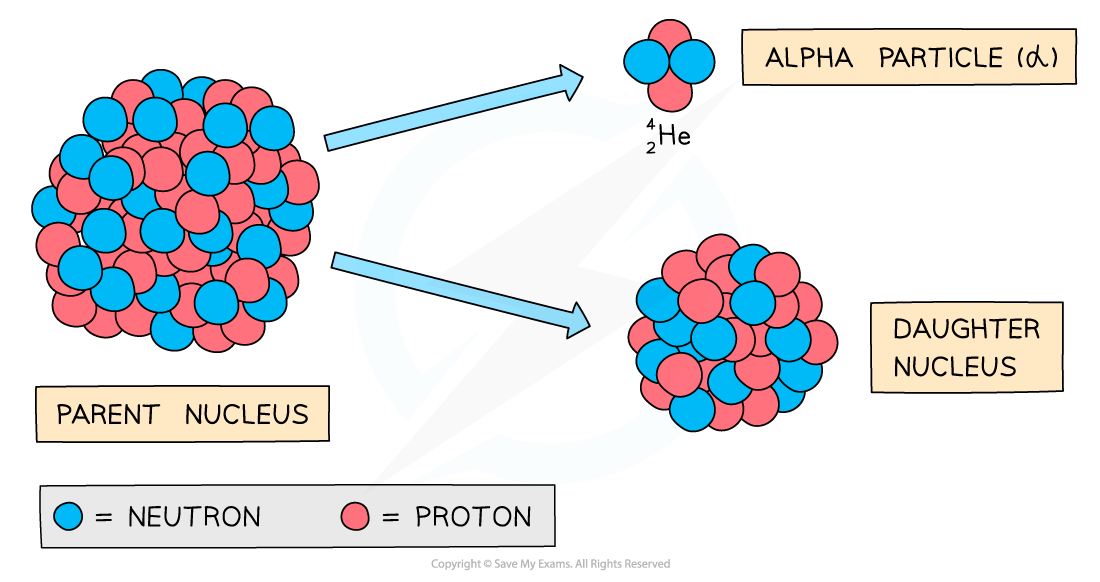
Alpha decay usually happens in large unstable nuclei, causing the overall mass and charge of the nucleus to decrease
An alpha particle is a helium nucleus
It is made of 2 protons and 2 neutrons
When the alpha particle is emitted from the unstable nucleus, the mass number and atomic number of the nucleus changes
The mass number decreases by 4
The atomic number decreases by 2
The charge on the nucleus also decreases by 2
This is because protons have a charge of +1 each
Beta Decay
During beta decay, a neutron changes into a proton and an electron
The electron is emitted and the proton remains in the nuclei
A completely new element is formed because the atomic number changes

Beta decay often happens in unstable nuclei that have too many neutrons. The mass number stays the same, but the atomic number increases by one
A beta particle is a high-speed electron
It has a mass number of 0
This is because the electron has a negligible mass, compared to neutrons and protons
Therefore, the mass number of the decaying nuclei remains the same
Electrons have an atomic number of -1
This means that the new nuclei will increase its atomic number by 1 in order to maintain the overall atomic number before and after the decay
Gamma Decay
During gamma decay, a gamma ray is emitted from an unstable nucleus
The process that makes the nucleus less energetic but does not change its structure

Gamma decay does not affect the mass number or the atomic number of the radioactive nucleus, but it does reduce the energy of the nucleus
The gamma ray that is emitted has a lot of energy, but no mass or charge

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
