Atomic Nuclei (OCR GCSE Physics A (Gateway)): Revision Note
Exam code: J249
Atomic Nuclei
Atoms are extremely small with a radius of about 1 x 10-10 metres
Atoms have a tiny, dense nucleus at their centre, with electrons orbiting around the nucleus
The central nucleus contains protons and neutrons only which are packed close together in a small region of space
Because protons are positive and neutrons are neutral, the charge of the nucleus is positive
The number of protons in the nucleus determines the element
Therefore the nucleus of each element contains a characteristic positive charge
e.g. a helium nucleus, with 2 protons, has a charge of +2
e.g. a uranium nucleus, with 92 protons, has a charge of +92
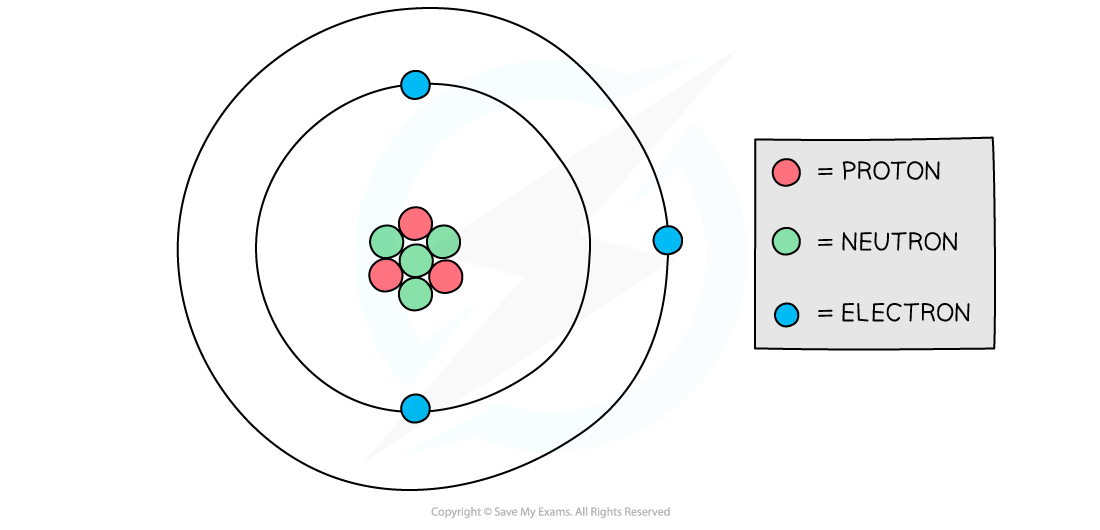
Diagram showing the structure of a Lithium atom. If drawn to scale then the electrons would be around 100 metres away from the nucleus!
The radius of the nucleus is about 10 000 times smaller than that of the atom, so it is an extremely small region of space compared to the overall size of the atom
This means that rather than being evenly spread out throughout the atom, virtually all of the atom's mass is concentrated inside the nucleus
Electron Energy Levels
Electrons in an atom orbit around the nucleus at particular distances, known as energy levels
A certain number of electrons can occupy each energy level
For example, only two electrons can orbit in the first energy level
Only eight electrons can fit in the second energy level, and eight in the third as well
The higher the energy level, the further the distance of the electron from the nucleus

In this diagram the first two energy levels are full. Electrons further from the nucleus have more energy
Like moving up a ladder, electrons in higher energy levels have greater potential energy because they have more distance between them and the nucleus
Electrons can move between energy levels by absorbing or emitting electromagnetic radiation
Energy is required for an electron to move from a lower to a higher energy level
This transition is called an excitation
Energy is released if the electron moves from a higher to a lower energy level
This transition is called a de–excitation
Ionisation
If an atom gains or loses an electron from the outer energy level this is known as ionisation
The charged particle is now known as an ion
An ion is an atom or particle with a non-zero charge
Positive ions are formed when electrons in the outer energy level are knocked out from an atom
This is because an electron is negatively charged
Positive ionisation can happen in a number of ways:
When objects are rubbed together, electrons can be removed by friction
When electrons absorb electromagnetic radiation they can gain enough energy to leave the atom
From chemical reactions

When radiation passes close to atoms it can knock electrons out, leaving the atom with an overall positive charge
Negative ions are formed when the outer shell of an atom gains an electron
Negative ionisation most commonly occurs during chemical reactions
Examiner Tips and Tricks
If you are studying for your Chemistry GCSE then you will need to know the number of electrons that fit into the different energy levels. They may also be called electron shells.

Unlock more, it's free!
Was this revision note helpful?
