Heat Energy & Temperature (OCR GCSE Physics A (Gateway)): Revision Note
Exam code: J249
Heat Energy & Temperature
The molecules within a substance possess two forms of energy:
Kinetic energy (due to their random motion / vibration)
Potential energy (due to their position relative to each other)
Together, these two form the total energy that makes up the internal energy of the system
Internal energy is defined as:
The total energy stored inside a system by the particles that make up the system due to their motion and positions
Heating and Temperature Change
Heating a system changes a substance's internal energy by increasing the kinetic energy of its particles
The temperature of the material, therefore, is related to the average kinetic energy of the molecules
The higher the temperature, the higher the kinetic energy of the molecules and vice versa
This means they move around faster
This increase in kinetic energy (and therefore internal energy) can:
Cause the temperature of the system to increase
Or, produce a change of state (solid to liquid or liquid to gas)
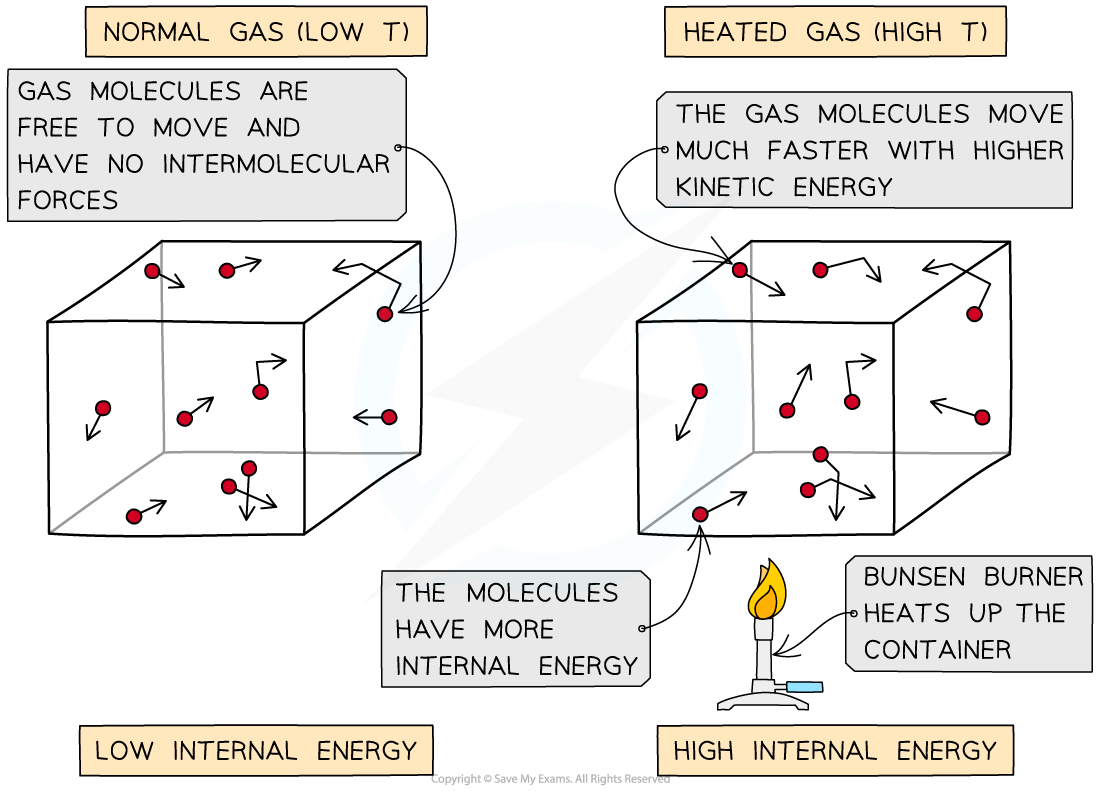
As the container is heated up, the gas molecules move faster with higher kinetic energy and therefore higher internal energy
Heating and Changes of State
When a substance reaches a certain temperature, the kinetic energy of the molecules will stop increasing and the energy will go into increasing its potential energy instead
This breaks the bonds between the molecules, causing them to move further apart and leads to a change of state
For example, liquid to gas
When a substance changes its state:
The potential energy of the molecules increases, breaking the bonds between them and becoming further apart
The kinetic energy remains the same, meaning that the temperature will remain the same, even though the substance is still being heated
Heating Curve
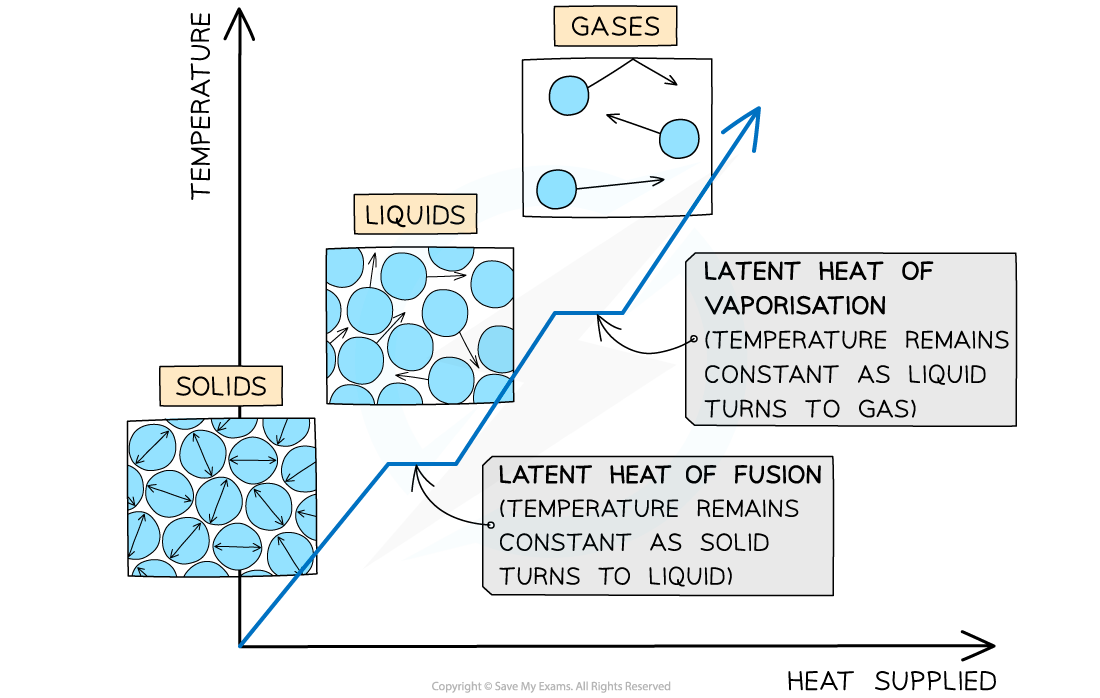
This graph shows how the temperature of a substance changes with time as it is heated
The substance is heated until it has melted to become a liquid, and then boiled to become a gas

Heating curve of a substance showing the energy changes as temperature is increased
The different sections of the graph show:
ORIGIN to A: Added heat energy is being used to increase the kinetic energy of the particles while it is a solid
A to B: Added heat energy is being used to break the bonds between the solid molecules, increasing the potential energy and melting the substance
B to C: Added heat energy is being used to further increase the kinetic energy of the particles while the substance is a liquid
C to D: Added heat energy is being used to break the bonds between the liquid molecules, further increasing the potential energy and boiling the substance
D to E: Added heat energy is being used to further increase the kinetic energy of the particles while the substance is a gas
Specific Heat Capacity vs Specific Latent Heat
Specific Heat Capacity
If the temperature of the system increases, the increase in temperature of this system depends on:
The mass of the substance heated
The type of material
The energy input to the system
The specific heat capacity of a substance is defined as:
The amount of energy required to raise the temperature of 1 kg of the substance by 1 °C
Different substances have different specific heat capacities
If a substance has a low specific heat capacity, it heats up and cools down quickly (ie. it takes less energy to change its temperature)
If a substance has a high specific heat capacity, it heats up and cools down slowly (ie. it takes more energy to change its temperature)
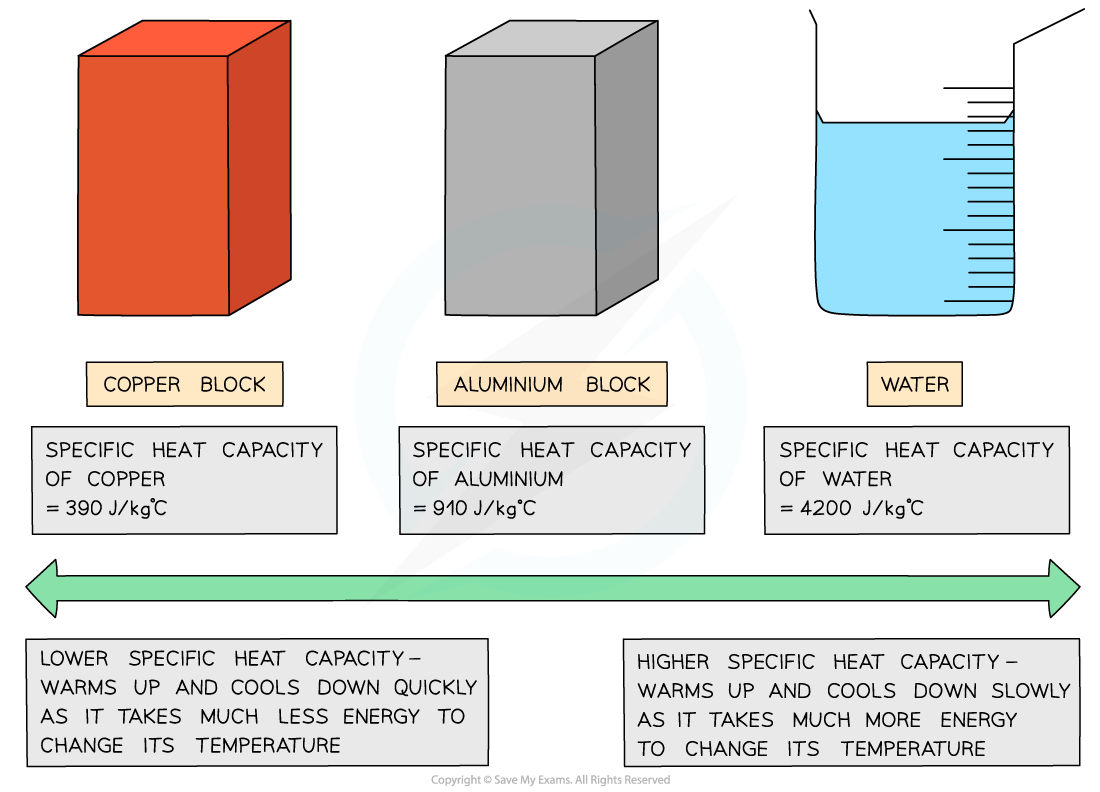
Low vs high specific heat capacity
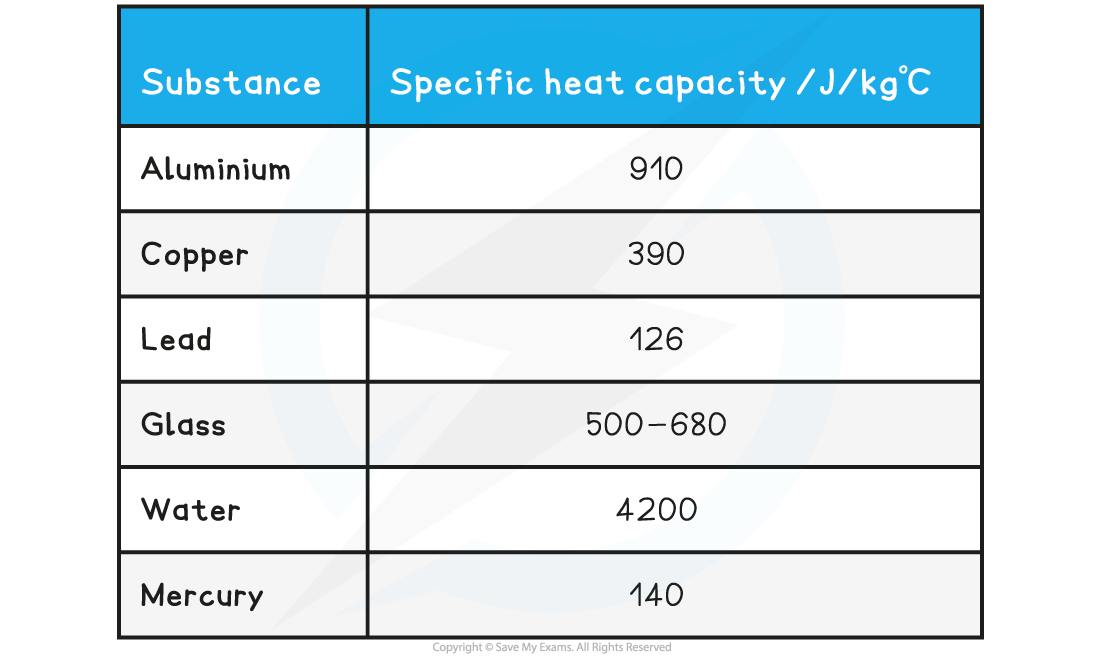
The specific heat capacity of some substances are given in the table below as examples:
Table of values of specific heat capacity for various substances
Specific Latent Heat
Energy is required to change the state of a substance
This energy is known as latent heat
The specific latent heat of a substance is defined as:
The amount of thermal energy required to change the state of 1 kg of a substance with no change in temperature
There are two types of specific latent heat:
Specific latent heat of fusion (solid to liquid and vice versa)
Specific latent heat of vaporisation (liquid to gas and vice versa)
Latent heat is represented by the symbol L with units joules per kilogram (J/kg)
Heat supplied against temperature graph, showing the changes in state for a substance
The specific latent heat of fusion is defined as:
The thermal energy required to convert 1 kg of solid to liquid with no change in temperature
This is used when melting a solid or freezing a liquid
When a solid substance melts, its temperature stays constant until all of the substance has become a liquid
The latent heat of fusion is the energy needed to break the bonds between the molecules
The specific latent heat of vaporisation is defined as:
The thermal energy required to convert 1 kg of liquid to gas with no change in temperature
This is used when vaporising a liquid or condensing a gas
When a liquid substance is heated up to its boiling point, the substance boils and turns into vapour
The latent heat of vaporisation is the energy needed by the particles to break away from their neighbouring particles in the liquid
Specific heat capacity and specific latent heat are slightly different
Specific heat capacity is used for a change in temperature in the same state
Specific latent heat is used for a change in state but no change in temperature

The difference between specific heat capacity and specific latent heat
Examiner Tips and Tricks
The specific latent heat of fusion and vaporisation value of all substances will be provided for you in the exam question, so you do not need to memorise any.
However, make sure to include 'with no change in temperature' in your definition of specific latent heat to be awarded full marks. Use these reminders to help you remember which type of latent heat is being referred to:
Latent heat of fusion = imagine ‘fusing’ the liquid molecules together to become a solid
Latent heat of vaporisation = “water vapour” is steam, so imagine vaporising the liquid molecules into a gas

Unlock more, it's free!
Did this page help you?