Electromagnetic (EM) Waves (OCR GCSE Physics A (Gateway)): Revision Note
Exam code: J249
Range of EM Waves
The relationship between frequency and wavelength of waves across the Electromagnetic spectrum is
The higher the frequency, the shorter the wavelength
The lower the frequency, the longer the wavelength
This means that radio waves have a lower frequency, and a longer wavelength than UV waves
This can be seen from the wave equation
v = fλ
Where:
v = speed of the wave in metres per second (m/s)
f = frequency of the wave in hertz (Hz)
λ = wavelength of the wave in metres (m)
Since all electromagnetic waves travel at the speed of light, c, this is constant
Therefore, in the equation, in keeping v constant, if f increases then λ must decrease
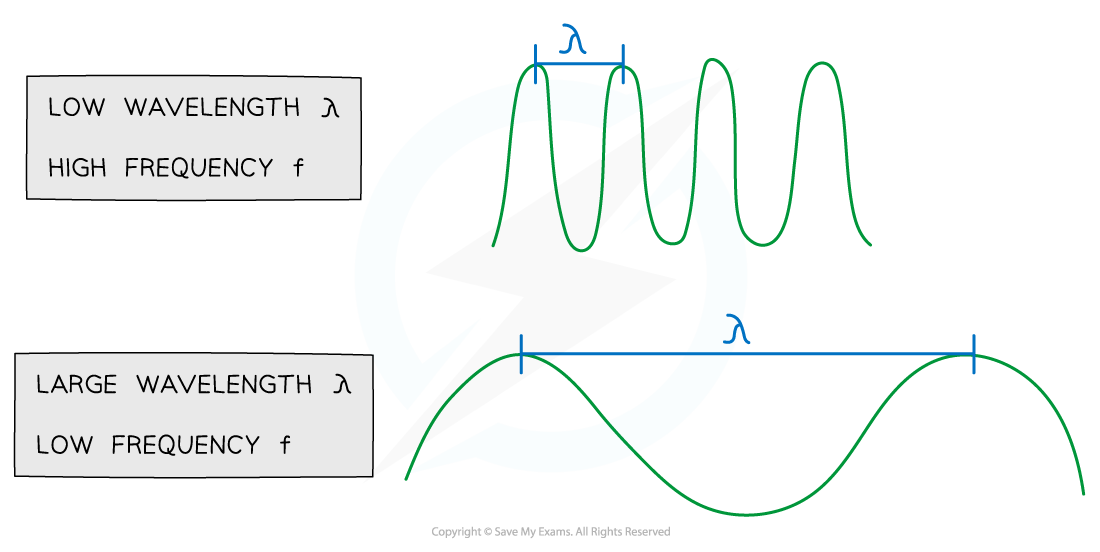
Relationship between wavelength and frequency
The higher the frequency, the higher the energy of electromagnetic radiation
Radiation with higher energy is:
Highly ionising
Harmful to cells and tissues causing cancer (e.g. UV, X-rays, Gamma rays)
Radiation with lower energy is:
Useful for communications
Less harmful to humans
Examiner Tips and Tricks
Make sure to remember this relation between wavelength and frequency and compare across the electromagnetic spectrum. High frequency, short wavelength waves are more dangerous than low frequency, long wavelength waves.
The EM Spectrum
The electromagnetic spectrum is arranged in a specific order based on the wavelengths or frequencies
The main groupings of the continuous electromagnetic (EM) spectrum are:
Radio waves
Microwaves
Infrared
Visible (red, orange, yellow, green, blue, indigo, violet)
Ultraviolet
X-rays
Gamma rays
This order is shown in the diagram below from longest wavelength (lowest frequency) to shortest wavelength (highest frequency)

Visible light is just one small part of a much bigger spectrum: The electromagnetic spectrum
Examiner Tips and Tricks
The electromagnetic spectrum is usually given in order of decreasing wavelength.
Remember:
Radios are big (long wavelength)
Gamma rays are emitted from atoms which are very small (short wavelength)
Visible Light
Visible light is defined as the range of wavelengths which are visible to humans
Visible light is the only part of the spectrum detectable by the human eye
However, it only takes up 0.0035% of the whole electromagnetic spectrum
In the natural world, many animals, such as birds, bees and certain fish, are able to perceive beyond visible light and can see infra-red and UV wavelengths of light
The different colours of waves correspond to different wavelengths:
Red has the longest wavelength (and the lowest frequency and energy)
Violet has the shortest wavelength (and the highest frequency and energy)

Colours of the visible spectrum with increasing wavelength
Wavelength and frequency are inversely proportional, this means that:
An increase in wavelength is a decrease in frequency (towards the red end of the spectrum)
A decrease in wavelength is an increase in frequency (towards the violet end of the spectrum)


The colours of the visible spectrum: red has the longest wavelength; violet has the shortest
Examiner Tips and Tricks
To remember the colours of the visible spectrum either remember:
The name “Roy G. Biv”
Or the saying “Richard Of York Gave Battle In Vain”

Unlock more, it's free!
Did this page help you?