Energy Stores & Transfers (Edexcel GCSE Physics) : Revision Note
Did this video help you?
Energy Stores
Energy is stored in objects in different energy stores
Energy Stores Table
Energy Store | Description |
|---|---|
Kinetic | Moving objects have energy in their kinetic store |
Gravitational | Objects gain energy in their gravitational potential store when they are lifted through a gravitational field |
Elastic | Objects have energy in their elastic potential store if they are stretched, squashed or bent |
Magnetic | Magnetic materials interacting with each other have energy in their magnetic store |
Electrostatic | Objects with charge (like electrons and protons) interacting with one another have energy in their electrostatic store |
Chemical | Chemical reactions transfer energy into or away from a substance's chemical store |
Nuclear | Atomic nuclei release energy from their nuclear store during nuclear reactions |
Thermal | All objects have energy in their thermal store, the hotter the object, the more energy it has in this store |
Did this video help you?
Energy Transfers
Energy Transfer Pathways
Energy is transferred between stores by different energy transfer pathways
The energy transfer pathways are:
Mechanical
Electrical
Heating
Radiation
These are described in the table below:
Energy Transfer Pathway Table
Transfer Pathway | Description |
|---|---|
Mechanical working | When a force acts on an object (e.g. pulling, pushing, stretching, squashing) |
Electrical working | A charge moving through a potential difference (e.g. current) |
Heating (by particles) | Energy is transferred from a hotter object to a colder one (e.g. conduction) |
(Heating by) radiation | Energy transferred by electromagnetic waves (e.g. visible light) |
An example of an energy transfer by heating is a hot coffee heating up cold hands
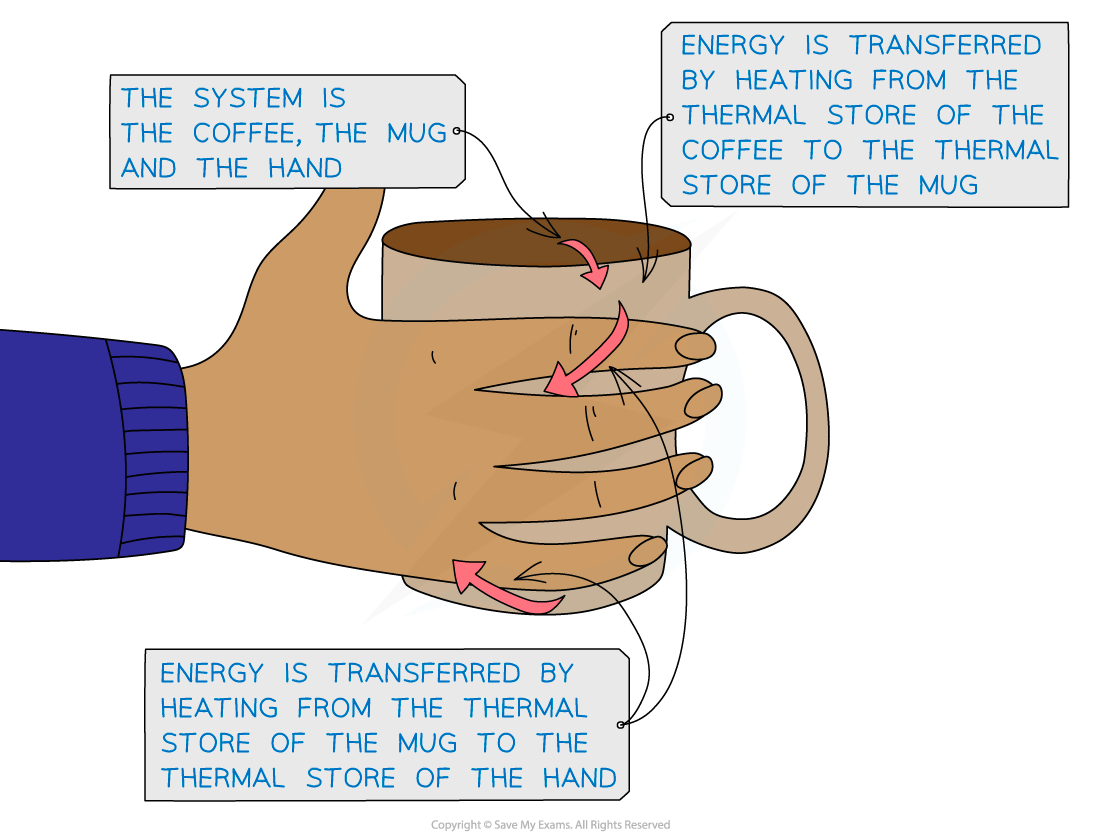
Energy is transferred by heating from the hot coffee to the mug to the cold hands
Energy Transfer Diagrams
Energy Flow Diagrams
Energy stores and transfers can be represented using a flow diagram
This shows both the stores and the transfers taking place within a system
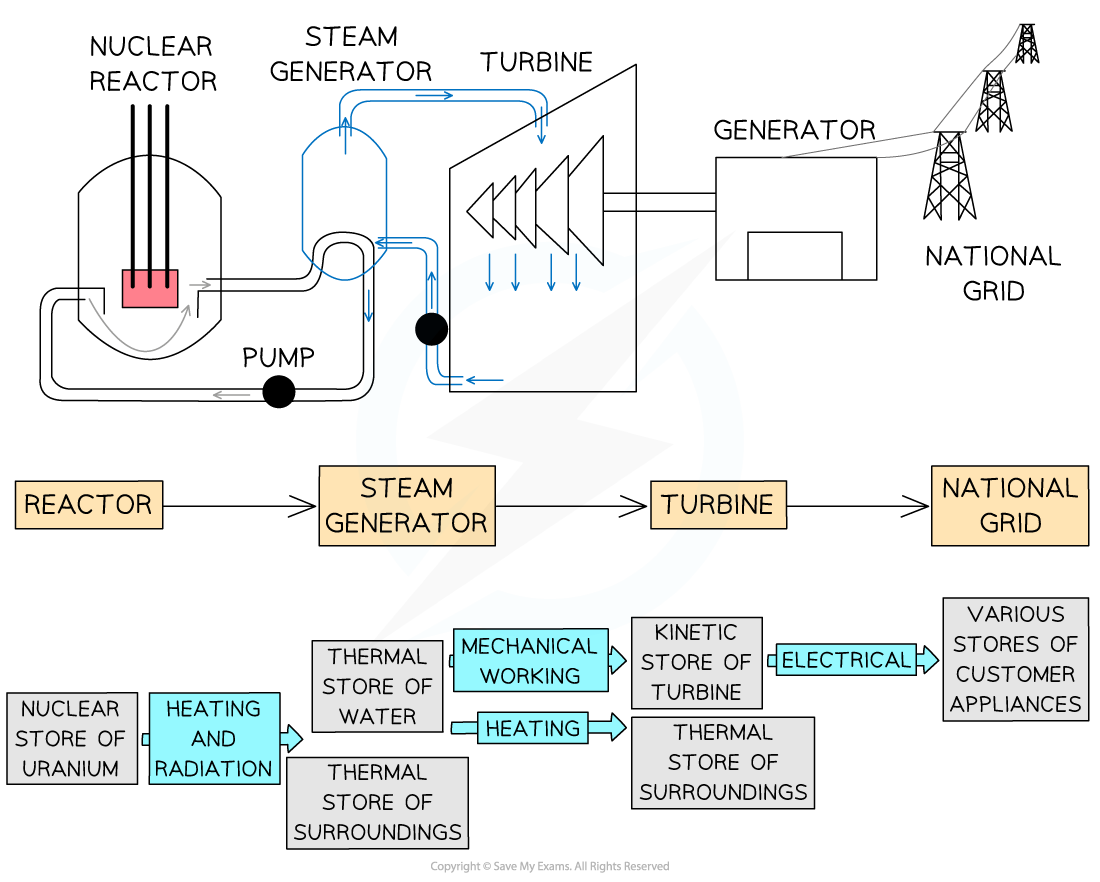
Energy flow diagram showing energy stores and transfers in a nuclear power plant.
Note the colour difference of the labels (stores) and the arrows (transfer pathways)
Sankey Diagrams
Sankey diagrams can be used to represent energy transfers
Sankey diagrams are characterised by the splitting arrows that show the proportions of the energy transfers taking place
The different parts of the arrow in a Sankey diagram represent the different energy transfers:
The left-hand side of the arrow (the flat end) represents the energy transferred into the system
The straight arrow pointing to the right represents the energy that ends up in the desired store; this is the useful energy output
The arrows that bend away represent the wasted energy
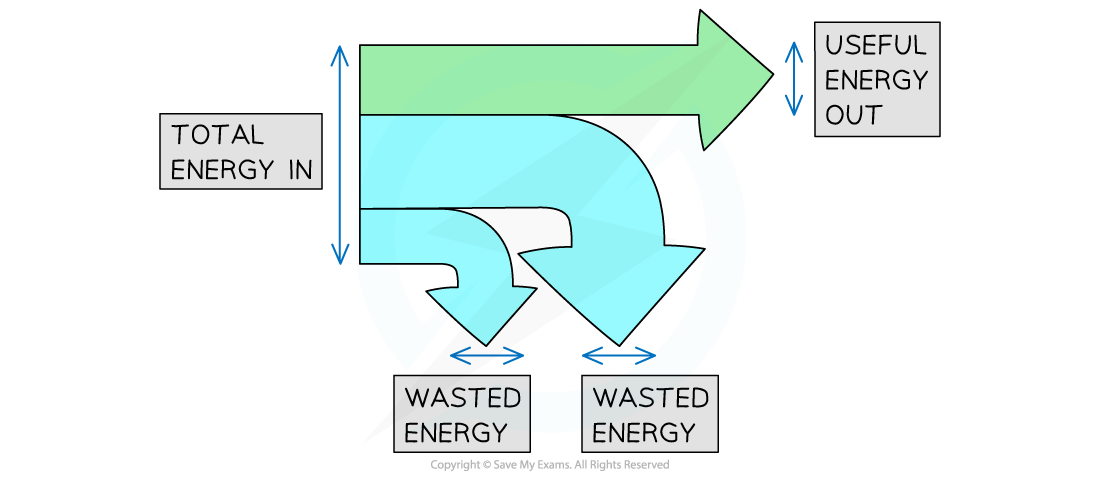
Total energy in, wasted energy and useful energy out shown on a Sankey diagram
The width of each arrow is proportional to the amount of energy being transferred As a result of the conversation of energy:
Total energy in = Useful energy out + Wasted energy
A Sankey diagram for a modern efficient light bulb will look very different from that for an old filament light bulb
A more efficient light bulb has less wasted energy
This is shown by the smaller arrow downwards representing the heat energy
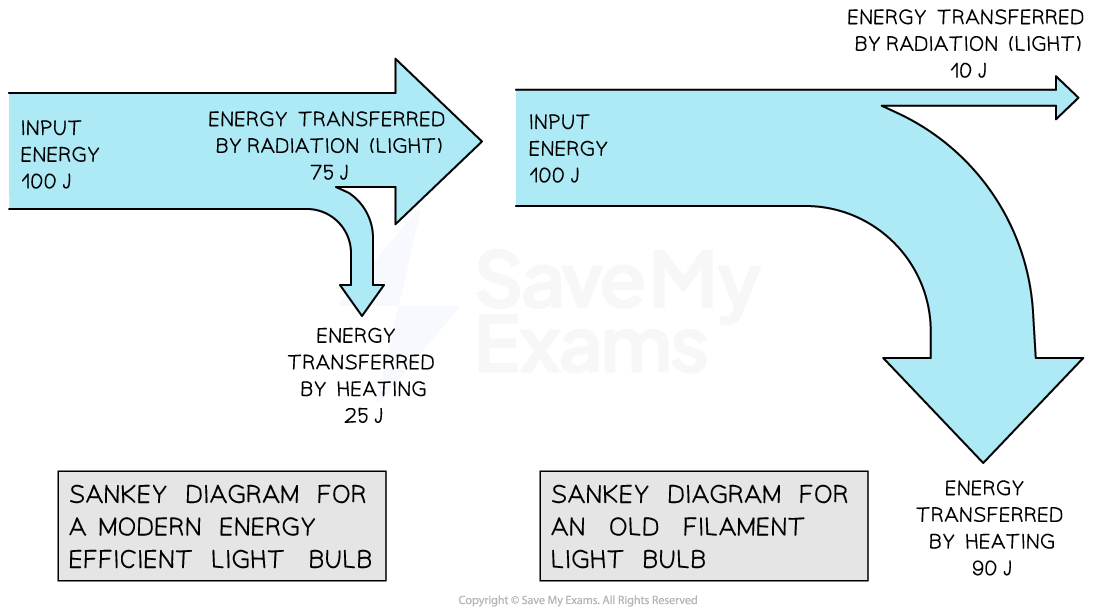
Sankey diagram for modern vs. old filament light bulb
Worked Example
An electric motor is used to lift a weight. The diagram represents the energy transfers in the system.

Calculate the amount of wasted energy.
Answer:
Step 1: State the conservation of energy
Energy cannot be created or destroyed, it can only be transferred from one store to another
This means that:
total energy in = useful energy out + wasted energy out
Step 2: Rearrange the equation for the wasted energy
wasted energy = total energy in – useful energy out
Step 3: Substitute the values from the diagram
500 – 120 = 380 J
Closed Systems & Energy Conservation
Systems
In physics, a system is defined as:
An object or group of objects
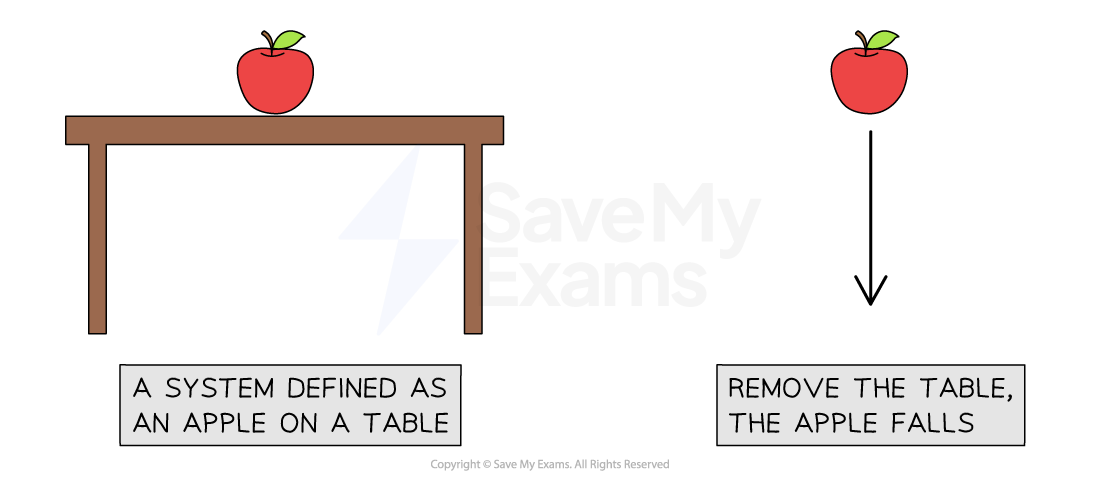
An apple sitting on a table can be defined as a system
Defining the system in physics is a way of narrowing the parameters to focus only on what is relevant to the situation being observed
When a system is in equilibrium, nothing changes and so nothing happens
When there is a change in a system, things happen, and when things happen energy is transferred
If the table is removed, the apple will fall
As the apple falls, energy is transferred
Energy is measured in units of joules (J)
A thermodynamic system, for example, can be isolated, closed or open
An open system allows the exchange of energy and matter to or from its surroundings
A closed system can exchange energy but not matter to or from its surroundings
An isolated system does not allow the transfer of matter or energy to or from its surroundings

A system can be open, closed or isolated
Conservation of Energy
The principle of conservation of energy states that:
Energy cannot be created or destroyed, it can only be transferred from one store to another
This means the total amount of energy in a closed system remains constant
The total energy transferred into a system must be equal to the total energy transferred out of the system
Therefore, energy is never 'lost' but it can be transferred to the surroundings
Energy can be dissipated (spread out) to the surroundings by heating and radiation
Dissipated energy transfers are often not useful, and can then be described as wasted energy
Example 1: A Bat Hitting a Ball
The moving bat has energy in its kinetic store
Some of that energy is transferred usefully to the kinetic store of the ball
Some of that energy is transferred from the kinetic store of the bat to the thermal store of the ball mechanically due to the impact of the bat on the ball
Some of that energy is dissipated by heating to the thermal store of the bat, the ball, and the surroundings


Energy transfers taking place when a bat hits a ball
Example 2: Boiling Water in a Kettle
When an electric kettle boils water, energy is transferred electrically from the mains supply to the thermal store of the heating element inside the kettle
As the heating element gets hotter, energy is transferred by heating to the thermal store of the water
Some of the energy is transferred to the thermal store of the plastic kettle (wasted energy transfer)
And some energy is dissipated to the thermal store of the surroundings due to the air around the kettle being heated (wasted energy transfer)

Energy transfer taking place as a kettle boils water

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
