Nuclear Equations (Edexcel GCSE Physics) : Revision Note
Did this video help you?
Nuclear Equations
Use given data to balance nuclear equations in terms of mass and charge
Nuclear radioactive decay equations show the changes in mass and charge of the nuclei in the decay
Each term will have the chemical symbol of the element or the type of radiation
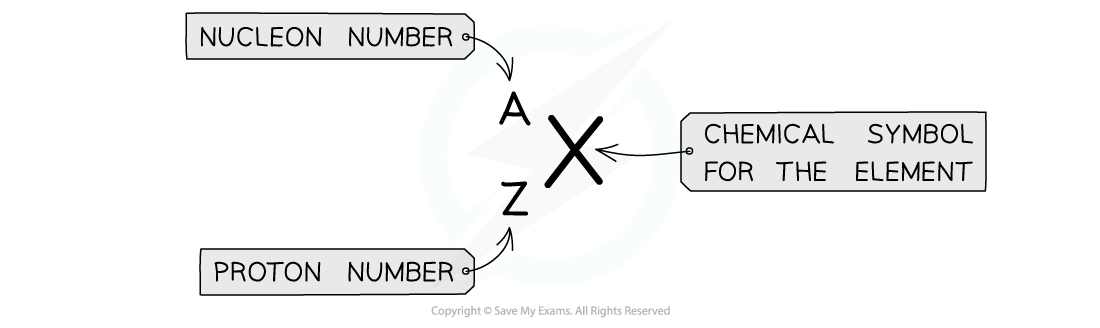
Nuclear notation
The top number A represents the nucleon number or the mass number
Nucleon number (A) = total number of protons and neutrons in the nucleus
The lower number Z represents the proton or atomic number
Proton number (Z) = total number of protons in the nucleus
Nuclear equations, just like chemical equations, balance:
The sum of the nucleon (mass) numbers on the left of each equation should equal the sum on the right
The sum of the proton (atomic) numbers should also balance on the left and right
The parent nucleus is the nucleus that decays
Subsequently, the daughter nucleus remaining after the decay
Alpha Decay Equation
In nuclear equations representing alpha decay:
The nucleon number of the daughter nucleus is 4 less than the parent
The proton number of the daughter nucleus is 2 less than the parent

Alpha decay equation
Beta Minus Decay Equation
In nuclear equations representing beta minus decay:
The nucleon number of the daughter nucleus is the same as the parent
The proton number of the daughter nucleus is 1 more than the parent

Beta-minus decay equation
Beta Plus Decay Equation
In nuclear equations representing beta plus decay:
The nucleon number of the daughter nucleus is the same as the parent
The proton number of the daughter nucleus is 1 less than the parent

Beta-plus decay equation
Gamma Decay Equation
In nuclear equations representing gamma decay:
The nucleon number of the daughter nucleus is the same as the parent
The proton number of the daughter nucleus is the same as the parent

Gamma decay equation
Worked Example

Answer: D


You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
