Pressure in Fluids (Edexcel GCSE Physics): Revision Note
Exam code: 1PH0
Pressure in a Fluid
A fluid is either a liquid or a gas
When an object on the Earth's surface is immersed in a liquid, the liquid exerts a pressure upon the object
This pressure is in addition to the pressure already exerted by the atmosphere
For example, an object at sea level (on the surface of the sea) experiences a pressure of 101 kPa due to the atmosphere
If this object is now immersed to a depth of 10 metres underwater, it experiences an extra pressure of 100 kPa due to the water
This means that the object will experience a total pressure of
101 kPa + 100 kPa = 201 kPa
This fluid pressure arises due to both:
The water (liquid) pressure
Atmospheric (gas) pressure
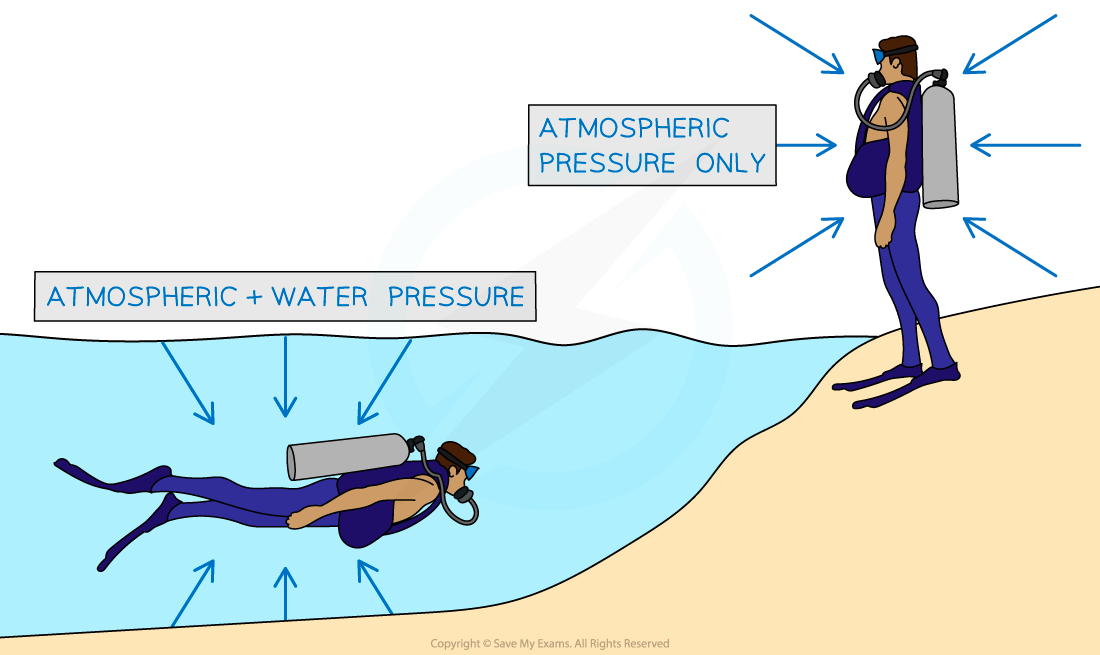
When an object is immersed in a liquid, it experiences pressure due to both the liquid and the atmosphere
The Force Exerted by a Fluid
When an object is immersed in a fluid, the fluid will exert pressure, squeezing the object
This pressure is exerted evenly across the whole surface of the fluid and in all directions
The pressure exerted on objects in fluids creates forces against surfaces
These forces act at 90 degrees (at right angles or 'normal') to the surface
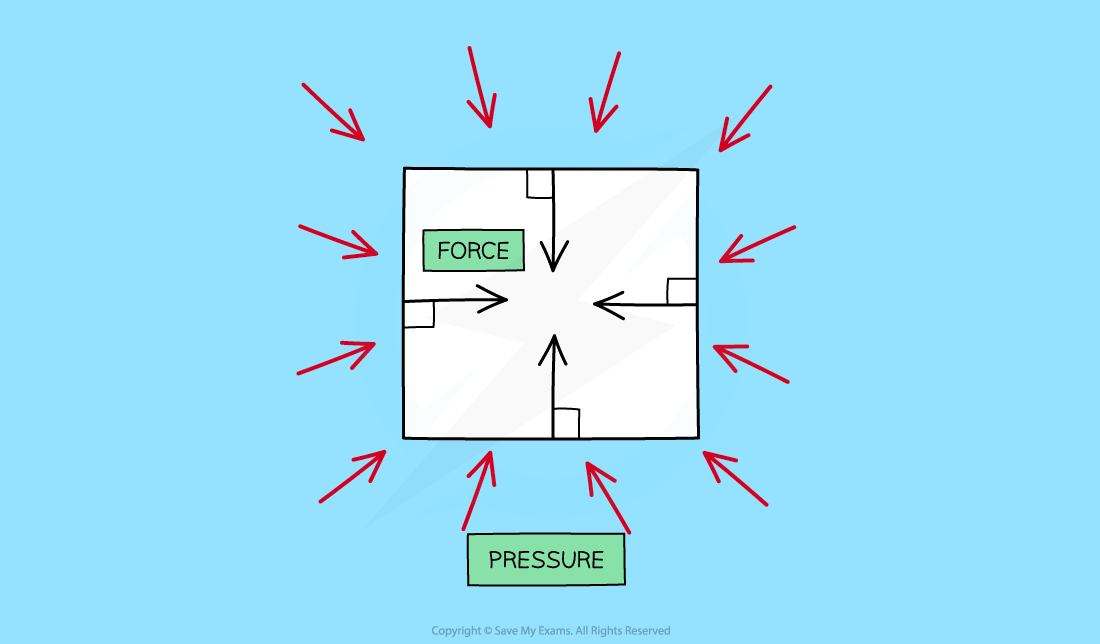
The pressure of a fluid on an object creates a force normal (at right angles) to the surface
Atmospheric Pressure
The Earth's atmosphere is a thin layer (relative to the size of the Earth) of air around it
It exerts a pressure of about 101 kPa at sea level

The Earth's atmosphere
The atmosphere extends more than 100 km into space and becomes less dense with increasing altitude (height above sea level)
This means that the pressure becomes less too
Atmospheric pressure various slightly from day to day, depending on the weather, and fine clear weather is usually associated with high pressure
The graph below shows how the pressure varies with altitude:
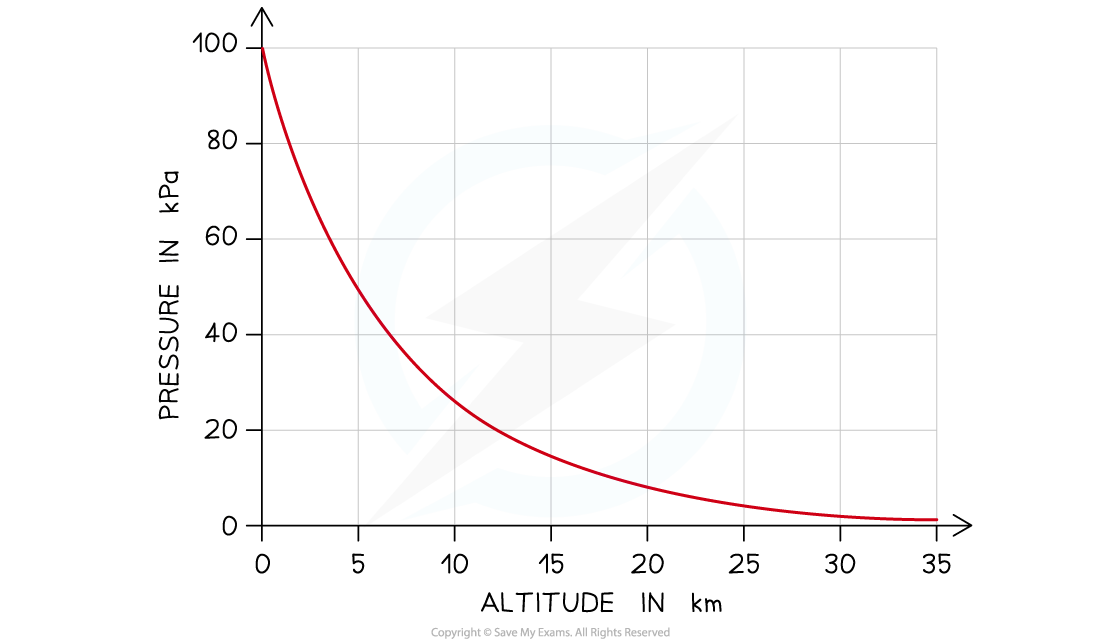
Graph of atmospheric pressure against altitude
Atmospheric pressure varies with height above a surface, for example, at sea level
This is due to air molecules colliding with a surface creating atmospheric pressure
These molecules create a force per area of the surface which creates the pressure
The number of air molecules (and so the weight of air) above a surface decreases as the height of the surface above ground level increases
This is also known as the density of the air
Therefore, as height increases, there is always less air above a surface than there is at a lower height and the atmospheric pressure decreases with an increase in height
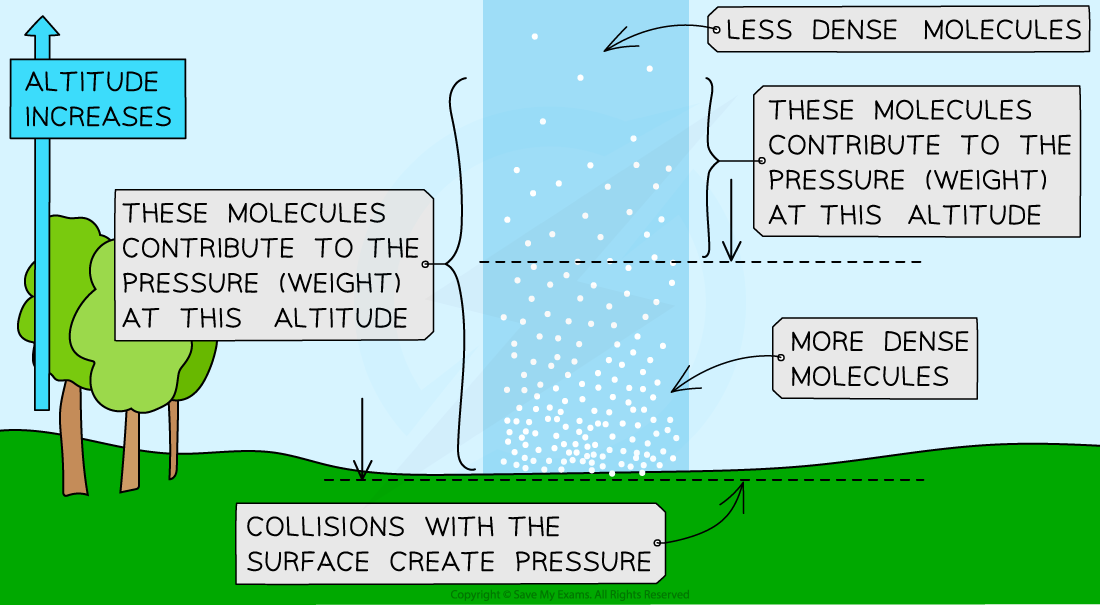
Atmospheric pressure decreases as the density of the molecules decreases

Unlock more, it's free!
Did this page help you?