Pressure & Volume (Edexcel GCSE Physics) : Revision Note
Pressure Changes in a Gas
If the temperature of a gas remains constant, the pressure of the gas changes when it is:
Compressed – decreases the volume which increases the pressure
Expanded – increases the volume which decreases the pressure

Pressure increases when a gas is compressed
Similarly, a change in pressure can cause a change in volume
A vacuum pump can be used to remove the air from a sealed container
The diagram below shows the change in volume to a tied up balloon when the pressure of the air around it decreases:

Therefore, if the gas is compressed, the molecules will hit the walls of the container more frequently
This creates a larger overall net force on the walls which increases the pressure
Pressure on Surfaces
As the gas particles move about randomly they collide with the walls of their containers
These collisions produce a net force at right angles to the wall of the gas container (or any surface)
Therefore, a gas at high pressure has more frequent collisions with the container walls and a greater force
Hence the higher the pressure, the higher the force exerted per unit area

Gas molecules bouncing off the walls of a container
It is possible for someone to experience this force by closing their mouth and forcing air into their cheeks
The strain on the cheeks is due to the force of the gas particles pushing at right angles to the cheeks
Pressure and Volume
In a gas, the molecules are widely spread
This makes the gas easy to expand and compress
Changing the pressure acting on the gas will compress it or allow it to expand if the temperature is kept constant
When a gas is compressed, the volume is decreased
The density of the gas increases, since the size of the container has decreased but the number of molecules has remained the same
This allows more frequent collisions of the molecules on the container wall
This means they hit the walls with a greater force and therefore increases the pressure
When a gas expands, the volume is increased
This causes a decrease in pressure
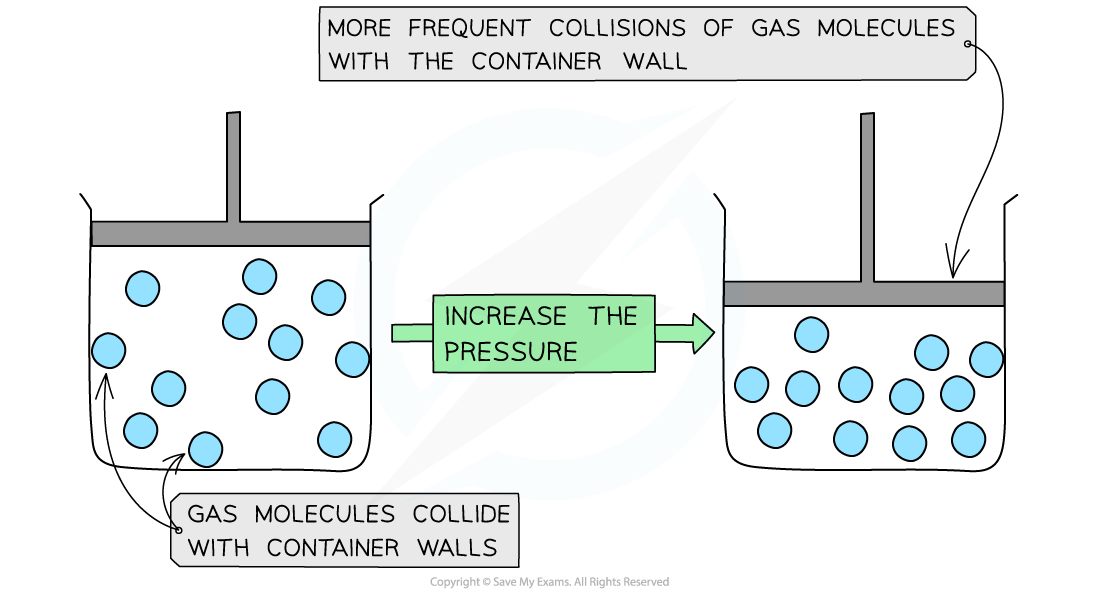
Decreasing the volume increases the pressure of molecules at the same temperature
Therefore, in summary:
When the volume decreases (compression), the pressure increases
When the volume increases (expansion), the pressure decreases
The key assumption is that the temperature and the mass (and number) of the particles remains the same
Worked Example
A deodorant can contains a highly pressurised fluid that pushes the deodorant out as a fine mist.

Which of the following is not a true statement about this situation?
A. The total number of particles remains constant throughout
B. The temperature of the deodorant remains constant throughout
C. The pressure of the deodorant decreases as it leaves the can
D. The total volume of the deodorant increases as it leaves the can
Answer: B
A is true because the particles only spread about, but there is no chemical change
C is true because the particles have a larger volume, which means the collide less frequently with any surfaces
the pressure therefore decreases
D is true because the deodorant is able to spread out as it leaves the can
B is not true because as gases expand their temperatures decrease

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
