Kinetic Theory (Edexcel GCSE Physics) : Revision Note
Did this video help you?
The Pressure of a Gas
Motion of Particles in a Gas
Molecules in a gas are in constant random motion at high speeds
Random motion means that the molecules are travelling in no specific path and undergo sudden changes in their motion if they collide:
With the walls of its container
With other molecules
The random motion of tiny particles in a fluid is known as Brownian motion

Random motion of gas molecules in a container
Pressure
Molecules of gas in a container will collide with the container walls
Pressure is defined as the force exerted per unit area
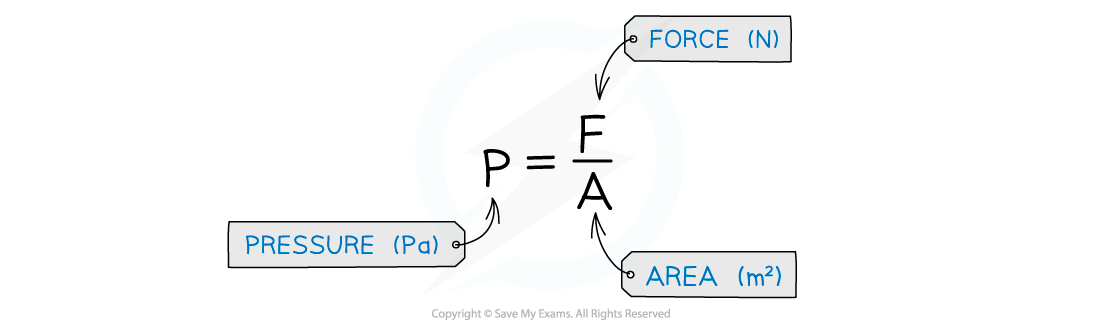
Pressure is measured in the units Pascals (Pa)
The area should always be the cross-sectional area of the object
This means the area where the force is at right angles to it
This equation can be rearranged with the help of a formula triangle:
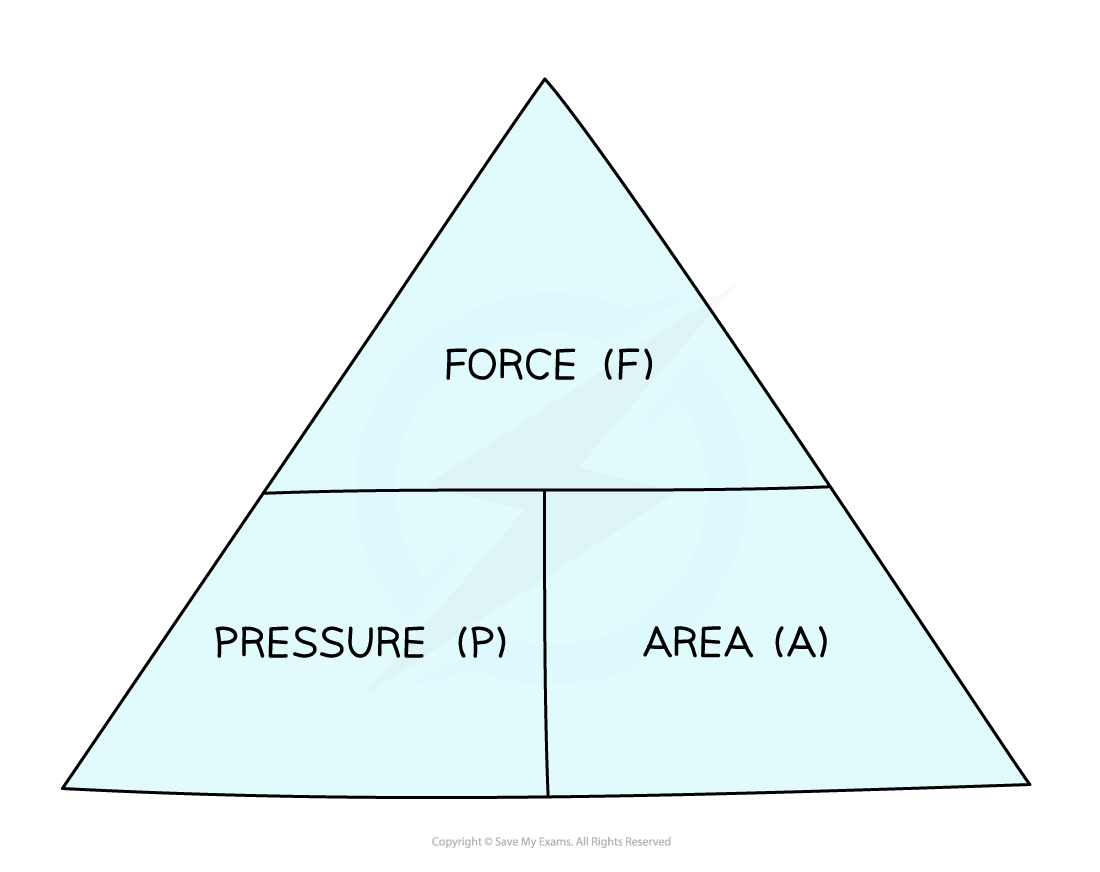
Pressure, force, area formula triangle
Imagine molecules of gas that are free to move around in a box
The molecules in the gas move around randomly at high speeds, colliding with surfaces and exerting pressure upon them
The temperature of a gas is related to the average speed of the molecules:
The hotter the gas, the faster the molecules move and vice versa
Hence, the molecules collide with the surface of the walls more frequently and with more force
This increases the pressure
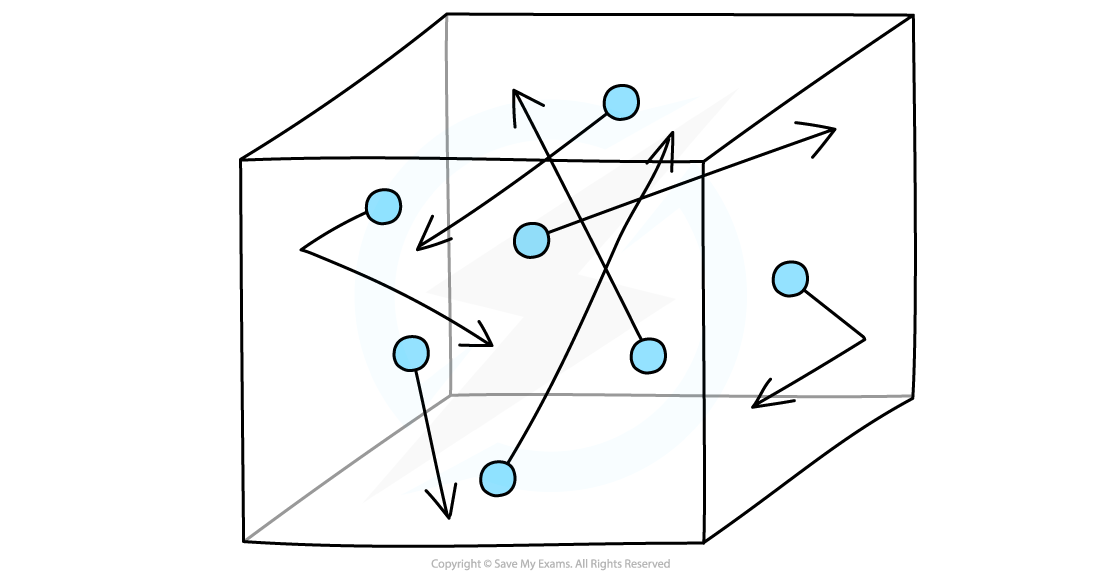
Gas molecules hit the sides of the container which creates pressure
Temperature & Pressure
The motion of molecules in a gas changes according to the temperature
As the temperature of a gas increases, the average speed of the molecules also increases
Since the average kinetic energy depends on their speed, the kinetic energy of the molecules also increases if its volume remains constant
The hotter the gas, the higher the average kinetic energy
The cooler the gas, the lower the average kinetic energy
If the gas is heated up, the molecules will travel at a higher speed
This means they will collide with the walls more often
This creates an increase in pressure
Therefore, at a constant volume, an increase in temperature increases the pressure of a gas and vice versa
Diagram A shows molecules in the same volume collide with the walls of the container more with an increase in temperature
Diagram B shows that since the temperature is proportional to the pressure, the graph against each is a straight line

At constant volume, an increase in the temperature of the gas increases the pressure due to more collisions on the container walls
Examiner Tips and Tricks
You are required to be able to describe the link between temperature and pressure qualitatively. This means that the correct use of terms such as 'collision', 'kinetic energy' and 'frequency', will be really important.

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
