Thermal Energy (Edexcel GCSE Physics): Revision Note
Exam code: 1PH0
Energy, Temperature & Changes of State
The molecules within a substance possess two forms of energy:
Kinetic energy (due to their random motion / vibration)
Potential energy (due to their position relative to each other)
Together, these two form the total energy that makes up the internal energy of the system
Internal energy is defined as:
The total energy stored inside a system by the particles that make up the system due to their motion and positions
Heating and Temperature Change
Heating a system changes a substance's internal energy by increasing the kinetic energy of its particles
The temperature of the material, therefore, is related to the average kinetic energy of the molecules
The higher the temperature, the higher the kinetic energy of the molecules and vice versa
This means they move around faster
This increase in kinetic energy (and therefore internal energy) can:
Cause the temperature of the system to increase
Or, produce a change of state (solid to liquid or liquid to gas)
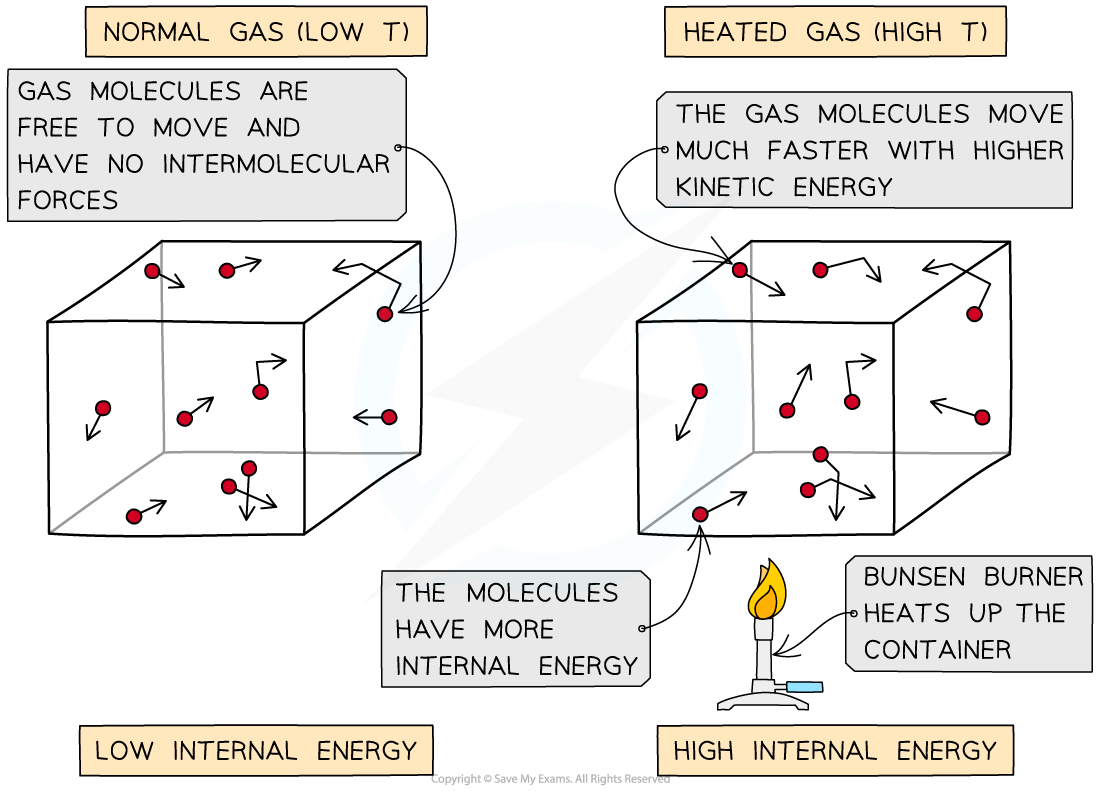
As the container is heated up, the gas molecules move faster with higher kinetic energy and therefore higher internal energy
Heating and Changes of State
When a substance reaches a certain temperature, the kinetic energy of the molecules will stop increasing and the energy will go into increasing its potential energy instead
This breaks the bonds between the molecules, causing them to move further apart and leads to a change of state
For example, liquid to gas
When a substance changes its state:
The potential energy of the molecules increases, breaking the bonds between them and becoming further apart
The kinetic energy remains the same, meaning that the temperature will remain the same, even though the substance is still being heated
Heating Curve
This graph shows how the temperature of a substance changes with time as it is heated
The substance is heated until it has melted to become a liquid, and then boiled to become a gas

Heating curve of a substance showing the energy changes as temperature is increased
The different sections of the graph show:
ORIGIN to A: Added heat energy is being used to increase the kinetic energy of the particles while it is a solid
A to B: Added heat energy is being used to break the bonds between the solid molecules, increasing the potential energy and melting the substance
B to C: Added heat energy is being used to further increase the kinetic energy of the particles while the substance is a liquid
C to D: Added heat energy is being used to break the bonds between the liquid molecules, further increasing the potential energy and boiling the substance
D to E: Added heat energy is being used to further increase the kinetic energy of the particles while the substance is a gas

Unlock more, it's free!
Did this page help you?