Alpha Decay (AQA GCSE Physics) : Revision Note
Alpha Decay
During alpha decay an alpha particle is emitted from an unstable nucleus
A completely new element is formed in the process
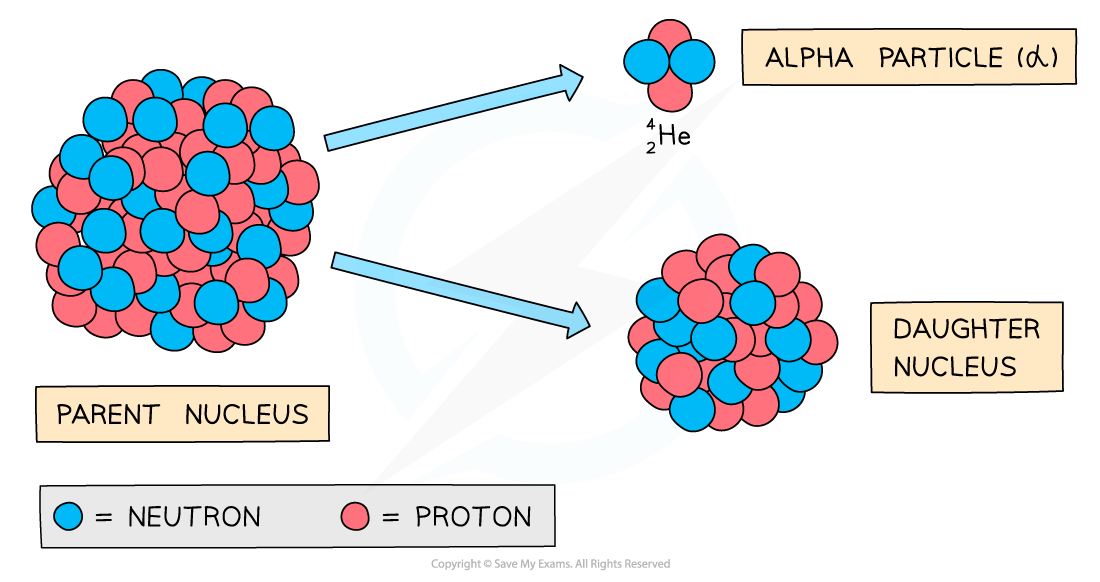
Alpha decay usually happens in large unstable nuclei, causing the overall mass and charge of the nucleus to decrease
An alpha particle is a helium nucleus
It is made of 2 protons and 2 neutrons
When the alpha particle is emitted from the unstable nucleus, the mass number and atomic number of the nucleus changes
The mass number decreases by 4
The atomic number decreases by 2
The charge on the nucleus also decreases by 2
This is because protons have a charge of +1 each
Decay Equations
The process of alpha decay can be shown as a decay equation
A decay equation is similar to a chemical reaction equation
The particles present before the decay are shown before the arrow
The particles produced in the decay are shown after the arrow
During decay equations, the sum of the mass and atomic numbers before the reaction must be the same as the sum of the mass and atomic numbers after the reaction
The following equation shows Polonium-212 undergoing alpha decay
It forms Lead-208 and an alpha particle
An alpha particle can also be written as a helium nucleus (Symbol He)

The polonium nucleus emits an alpha particle, causing its mass and charge to decrease. This means it changes into a new element
Worked Example
A nucleus with 84 protons and 126 neutrons undergoes alpha decay. It forms lead, which has the element symbol Pb.

Which of the isotopes of lead pictured is the correct one formed during the decay?
Answer: A
Step 1: Calculate the mass number of the original nucleus
The mass number is equal to the number of protons plus the number of neutrons
The original nucleus has 84 protons and 126 neutrons
84 + 126 = 210
The mass number of the original nucleus is 210
Step 2: Calculate the new atomic number
The alpha particle emitted is made of two protons and two neutrons
Protons have an atomic number of 1, and neutrons have an atomic number of 0
Removing two protons and two neutrons will reduce the atomic number by 2
84 – 2 = 82
The new nucleus has an atomic number of 82
Step 3: Calculate the new mass number
Protons and neutrons both have a mass number of 1
Removing two protons and two neutrons will reduce the mass number by 4
210 – 4 = 206
The new nucleus has a mass number of 206
Examiner Tips and Tricks
It is easy to forget that an alpha particle is a helium nucleus. The two are interchangeable, so don’t be surprised to see either used in the exam. You are not expected to know the names of the elements produced during radioactive decays, but you do need to be able to calculate the mass and atomic numbers by making sure they are balanced on either side of the reaction.

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
