Atomic Structure (AQA GCSE Physics) : Revision Note
Did this video help you?
Atomic Structure
Atoms are the building blocks of all matter
They are incredibly small, with a radius of only 1 × 10-10 m
This means that about one hundred million atoms could fit side by side across your thumbnail
Atoms have a tiny, dense nucleus at their centre, with electrons orbiting around the nucleus
The radius of the nucleus is over 10,000 times smaller than the whole atom, but it contains almost all of the mass of the atom

Diagram showing the structure of a Lithium atom. If drawn to scale then the electrons would be around 100 metres away from the nucleus!
Parts of the Atom
The nucleus contains:
Protons - positively charged particles with a relative atomic mass of one unit
Neutrons – no charge, and also with a relative atomic mass of one unit
Almost all of the atom is empty space, but moving around the nucleus there are:
Electrons – negative charge with almost no mass (1/2000 the mass of a proton or neutron)
The properties of each of the particles are shown in the table below:

Examiner Tips and Tricks
There are many different models of the atom. As you progress through the topic you will discover that the atom can be described in many different ways, such as the Plum Pudding Model that is covered later, but for your exam, make sure to only use the model and descriptions described here!
Be careful with your terminology:
Atom = nucleus (proton and neutron) and electrons
Nucleus = protons and neutrons at the centre of the atom
Electron Structure
Electrons in an atom orbit around the nucleus at particular distances, known as energy levels
A certain number of electrons can occupy each energy level
For example, only two electrons can orbit in the first energy level
Only eight electrons can fit in the second energy level, and eight in the third as well
The higher the energy level, the further the distance of the electron from the nucleus

In this diagram the first two energy levels are full. Electrons further from the nucleus have more energy
Like moving up a ladder, electrons in higher energy levels have greater potential energy because they have more distance between them and the nucleus
Examiner Tips and Tricks
If you are studying for your Chemistry GCSE then you will need to know the number of electrons that fit into the different energy levels. They may also be called electron shells.
Electrons & Protons
Although atoms contain particles of different charge, the total charge within an atom is zero
This is because the number of electrons is equal to the number of protons
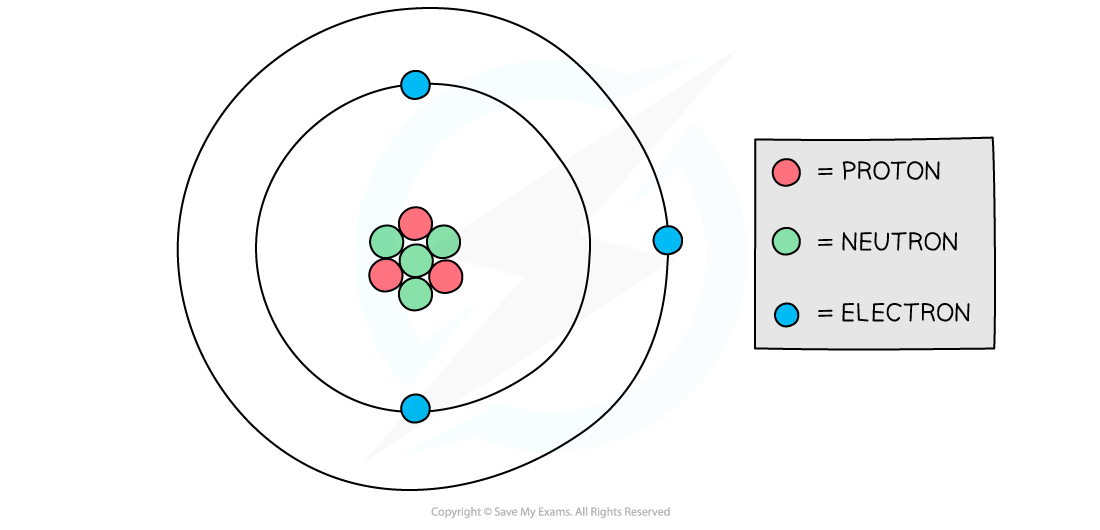
A Lithium atom has three protons, four neutrons and three electrons
The following table sets out the calculation of the total charge in the Lithium atom:
Total Charge Calculation Table

If an atom loses electrons, then it is said to be ionised
Worked Example
A nucleus of carbon-12 is shown below.

How many electrons are there in an atom of carbon-12?
Answer:
Step 1: Count the number of protons in the carbon nucleus
There are 6 protons in the carbon atom
Step 2: Determine the number of electrons
Remember, the number of electrons in an atom is equal to the number of protons
Therefore there must be 6 electrons in the carbon atom

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
