Required Practical: Investigating Specific Heat Capacity (AQA GCSE Physics) : Revision Note
Required Practical 1: Investigating Specific Heat Capacity
Aims of the Experiment
The aim of the experiment is to determine the specific heat capacity of a substance, by linking the amount of energy transferred to the substance with the rise in temperature of the substance
Variables:
Independent variable = Time, t
Dependent variable = Temperature, θ
Control variables:
Material of the block
Current supplied, I
Potential difference supplied, V
Equipment List

Resolution of measuring equipment:
Thermometer = 1 °C
Stopwatch = 0.01 s
Voltmeter = 0.1 V
Ammeter = 0.01 A
Method
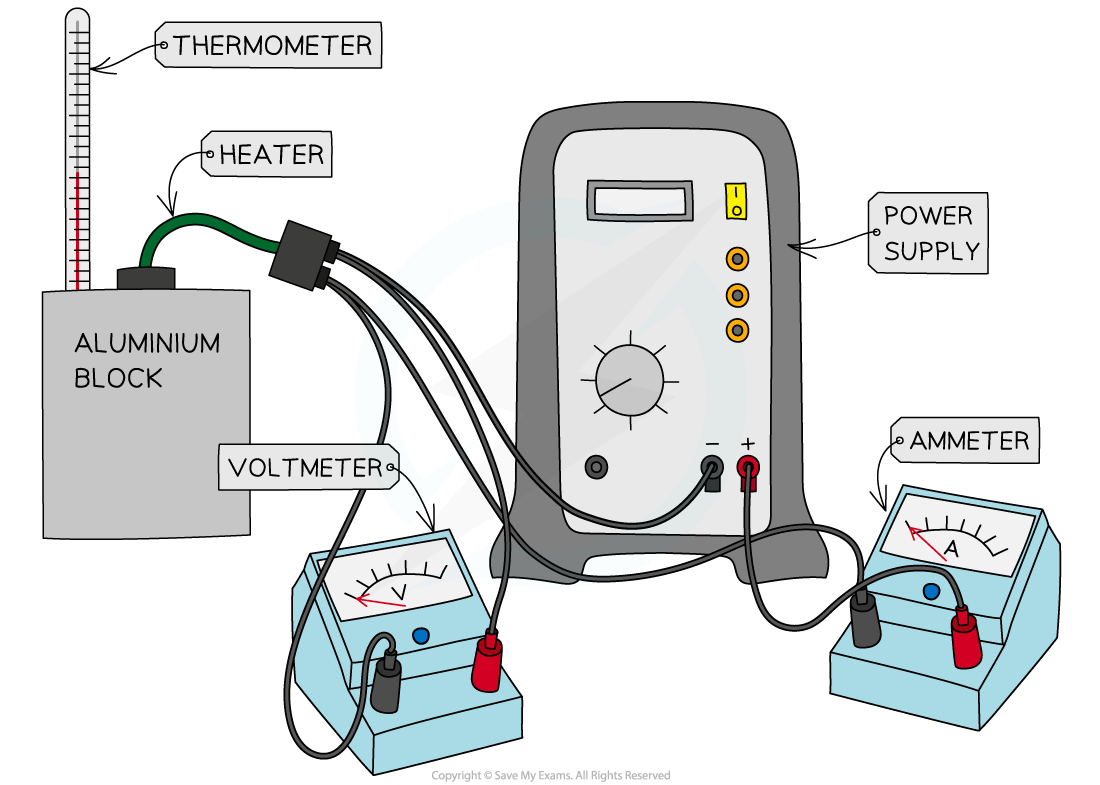
Apparatus to investigate the specific heat capacity of the aluminium block
Start by assembling the apparatus, placing the heater into the top of the block
Measure the initial temperature of the aluminium block from the thermometer
Turn on the power supply and start the stopwatch
Whilst the power supply is on, the heater will heat up the block. Take several periodic measurements, eg. every 1 minute of the voltage and current from the voltmeter and ammeter respectively, calculating an average for each at the end of the experiment up to 10 minutes
Switch off the power supply, stop the stopwatch and leave the apparatus for about a minute. The temperature will still rise before it cools
Monitor the thermometer and record the final temperature reached for the block
An example table of results might look like this:

Analysis of Results
The thermal energy supplied to the block can be calculated using the equations:
and
Where:
E = thermal energy, in joules (J)
Q = Charge, in coulombs (C)
I = current, in amperes (A)
V = potential difference, in volts (V)
t = time, in seconds (s)
Combining the equations:
Rearrange to make Q the subject
Substitute into the Q = It equation
Rearrange to make E the subject
The change in thermal energy is defined by the equation:
Where:
ΔE = change in energy, in joules (J)
m = mass, in kilograms (kg)
c = specific heat capacity, in joules per kilogram per degree Celsius (J/kg °C)
Δθ = change in temperature, in degrees Celsius (°C)
Rearranging for the specific heat capacity, c:
To calculate Δθ:
Where:
= final temperature
= initial temperature
To calculate ΔE:
Where:
I = average current, in amperes (A)
V = average potential difference (V)
θf = final time, in seconds (s)
θi = initial time, in seconds (s)
These values are then substituted into the specific heat capacity equation to calculate the specific heat capacity of the aluminium block
Evaluating the Experiment
Systematic Errors:
Make sure the voltmeter and ammeter are initially set to zero, to avoid zero error
Random Errors:
Not all the energy transferred from the heater will be transferred to the block, some will be dissipated to the surroundings into the surroundings and some will be transferred to the thermometer (also part of the surroundings)
This means the measured value of the specific heat capacity is likely to be higher than what it actually is
To reduce this effect, make sure the block is fully insulated
A joulemeter could be used to calculate energy directly
This would eliminate errors from the voltmeter, ammeter and the stopwatch
Make sure the temperature value is read at eye level from the thermometer, to avoid parallax error
The experiment can also be repeated with a beaker of water of equal mass, the water should heat up slower than the aluminium block
Safety Considerations
Make sure never to touch the heater whilst it is on, otherwise, it could burn skin or set something on fire
Run any burns immediately under cold running water for at least 5 minutes
Allow time for all the equipment, including the heater, wire and block to cool before packing away the equipment
Keep water away from all electrical equipment
Wear eye protection if using a beaker of hot water
Examiner Tips and Tricks
This practical investigation is usually done as a teacher demonstration because of the specialist equipment used. Therefore, students often find it more difficult to engage with than a hands-on practical, and so they can often find it quite confusing. This Required Practical does come up in exams very frequently, so you do need to make the extra effort to understand the equipment, the set-up, and how the measurements are taken.
The main idea is that the measurements of current and potential difference allow you to calculate the energy transferred to the metal block, and from there you can use the change in temperature and the energy supplied to calculate the specific heat capacity of the metal.

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
