Hydrogen Ions & pH (Edexcel GCSE Combined Science): Revision Note
Exam code: 1SC0
Hydrogen Ions & pH
We have already seen that acids are substances that contain hydrogen ions in solution
The more hydrogen ions the stronger the acid, but the lower the pH
The higher the concentration of hydroxide ions in a solution the higher the pH
So pH is a measure of the concentration of H+ ions in solution, but they have an inverse relationship
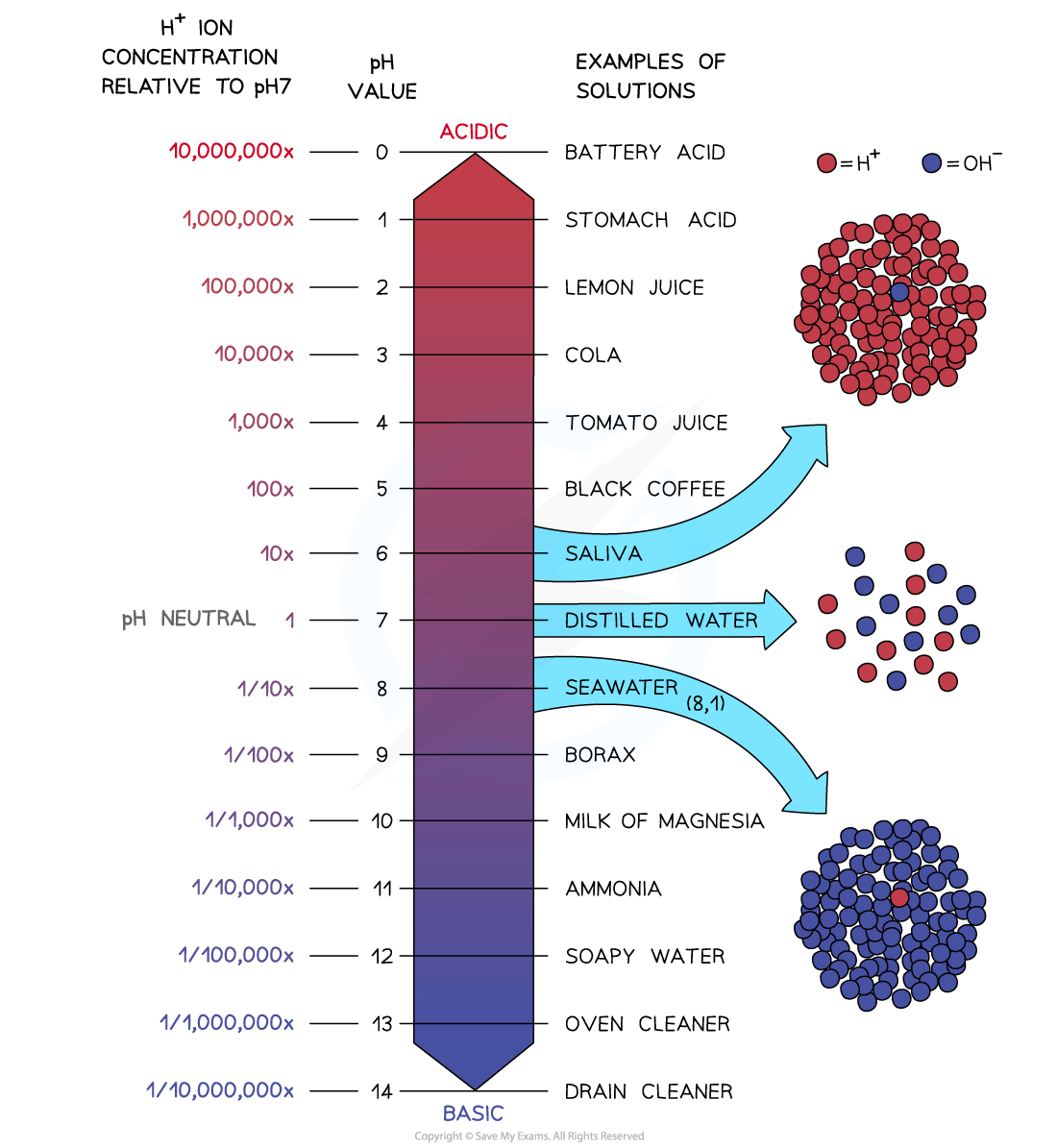
The pH scale is logarithmic so that each change in pH is a tenfold change in hydrogen ion concentration
The pH scale is logarithmic, meaning that each change of 1 on the scale represents a change in concentration by a factor of 10
Therefore an acid with a pH of 3 has ten times the concentration of H+ ions than an acid of pH 4
An acid with a pH of 2 has 10 x 10 = 100 times the concentration of H+ ions than an acid with a pH of 4
From this we can summarize that for two acids of equal concentration, where one is strong and the other is weak, then the strong acid will have a lower pH due to its capacity to dissociate more and hence put more H+ ions into solution than the weak acid
Examiner Tips and Tricks
Acid strength is reflected in how many hydrogen ions are in solution. The more hydrogen ions the lower the pH and vice-versa.

Unlock more, it's free!
Was this revision note helpful?
