Uses of Ethanol (WJEC GCSE Chemistry): Revision Note
Exam code: 3410
Alcoholic Drinks
Alcoholic drinks
Ethanol has been used in alcoholic drinks for thousands of years
Current alcohol consumption by the population is becoming as issue as it is readily available
The impacts fall into the following categories
Health
There are health issues associated with 'binge drinking' and misuse of alcohol over a long period
Over time excessive alcohol consumption can cause the following:
High blood pressure, heart disease, stroke, liver disease, and digestive problems
Cancer of the breast, mouth, throat, esophagus, voice box, liver, colon, and rectum
Weakened of the immune system
Learning and memory problems, including dementia
Mental health concerns
Alcohol dependence
Economic
Recent figures from the office of budget and responsibility state that duties from alcohol will raise £13.1 billion in 2023 - 2024
The exportation of alcoholic beverages also contributes £6 billion to the economy
The industry employs 650,000 people directly, and supports over 1 million more jobs in the wider economy
Despite the vast amount of money raised, alcohol misuse in the UK is estimated to cost over £21 billion per year as a result of healthcare costs, police costs and lost productivity due to missing work
Social
20 % of reports of violence take place close to pubs or clubs showing that alcohol has most likely played a part in these crimes
in the UK the annual cost of alcohol related crimes is between £8 billion and £13 billion
Alcoholic drinks

Is drinking too much alcohol bad for health?
Photo by NHS Better Health (opens in a new tab)
Solvents & Fuels
Other uses of ethanol
Solvents
A solvent is a substance which dissolves solutes
Diagram to show the process of dissolving
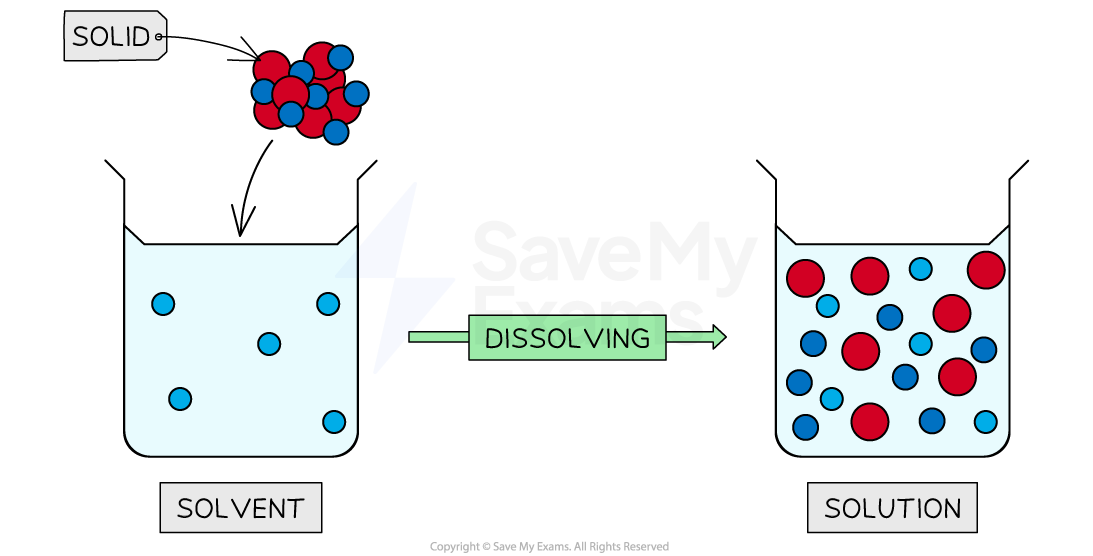
Using a solvent which dissolves the solute forms a solution
Ethanol can be used as a solvent to dissolve substances, including some that are insoluble in water
Methanol can also be be used as a solvent in the pharmaceutical industry and as antifreeze
Methanol is poisonous when ingested
Bioethanol
Bioethanol is a type of biofuel
Green plants absorb atmospheric carbon dioxide and convert it into glucose via photosynthesis:
carbon dioxide + water → glucose + oxygen
6CO2 (g) + 6H2O (l) → C6H12O6 (aq) + 6O2 (g)
The glucose is then further converted into ethanol by fermentation
The name bioethanol is simply identifying how the ethanol has been produced
The production of biofuel

The production of biofuels such as bioethanol is renewable
When biofuels are produced this way, they can be considered carbon neutral
This is because the carbon dioxide absorbed during photosynthesis equals the carbon dioxide produced by the combustion of the bioethanol
Table showing some advantages and disadvantages for use of bioethanol
Advantages of bioethanol | Disadvantages of bioethanol |
|---|---|
Carbon neutral | Bioethanol typically has lower specific energy than fossil fuels |
Renewable and sustainable if crops / trees are replanted | Many developed countries don't have the space to be able to produce enough plants to make bioethanol because the land is needed for food production |
Reduces greenhouse emissions / pollution | Conversion of engines and machinery to run on bioethanol instead of petrol / diesel |
Bioethanol production could provide money for less developed countries as they have the space to grow the crops required | Climate dependent to grow crops |
The production and use of bioethanol is considered to be carbon neutral
This does not take into account the carbon dioxide emissions from harvesting and transporting crops as well as the energy costs of producing the ethanol
Overall, more carbon dioxide is emitted than absorbed meaning this process contributes to global warming

Unlock more, it's free!
Was this revision note helpful?
