Investigating Electrolysis (WJEC GCSE Chemistry): Revision Note
Exam code: 3410
Core Practical: Electrolysis of Copper(II)Sulfate
Introduction
To electrolyse copper(II) sulfate solution using inert(graphite) electrodes followed by the use of copper electrodes
Apparatus
250 cm3 beaker
2 graphite electrodes
Beaker suitable for electrolysis
12 V DC. power supply
Leads and crocodile clips
400 cm3 copper(II) sulfate, about 0.5 mol dm–3
Diagram
Electrolysis of copper(II) sulfate using graphite electrodes

There are different observations for each electrode
Method
Graphite electrodes
Pour copper(II) sulfate solution into a beaker
Place two graphite rods into the copper sulfate solution
Attach one electrode to the negative terminal of a DC supply, and the other electrode to the positive terminal
Completely fill two small test tubes with copper(II) sulfate solution and position a test tube over each electrode as shown in the diagram
Turn on the power supply and observe what happens at each electrode
Test any gas produced with a glowing splint and a burning splint
Record observations of what happens at each electrode, including the results of the gas tests
Observations at anode | Observations at cathode |
|
|
Practical tip
The anode will always be the one where gas is produced, which means that you will see bubbles
Analysis of Results
Results table
Observations at anode | Observations at cathode |
Bubbling seen and gas formed relights a glowing splint | Orange / brown solid forming on electrode |
Evaluation
Copper metal is formed at the negative electrode and oxygen gas is formed at the positive electrode
Conclusion
Copper ions are attracted to the negative electrode and are reduced (gain electrons)
Cu2+ (aq) + 2e– → Cu (s)
At the positive electrode, oxygen gas is formed.
The equation for this reaction is:
2OH– (aq) → O2 (g) + 2H+ (aq) + 4e–
Using copper electrodes
Apparatus
250 cm3 beaker
2 copper electrodes
beaker suitable for electrolysis
12 V DC. power supply
Leads and crocodile clips
400 cm3 copper(II) sulfate, about 0.5 mol dm–3
Diagram
Electrolysis of copper sulfate using graphite electrodes
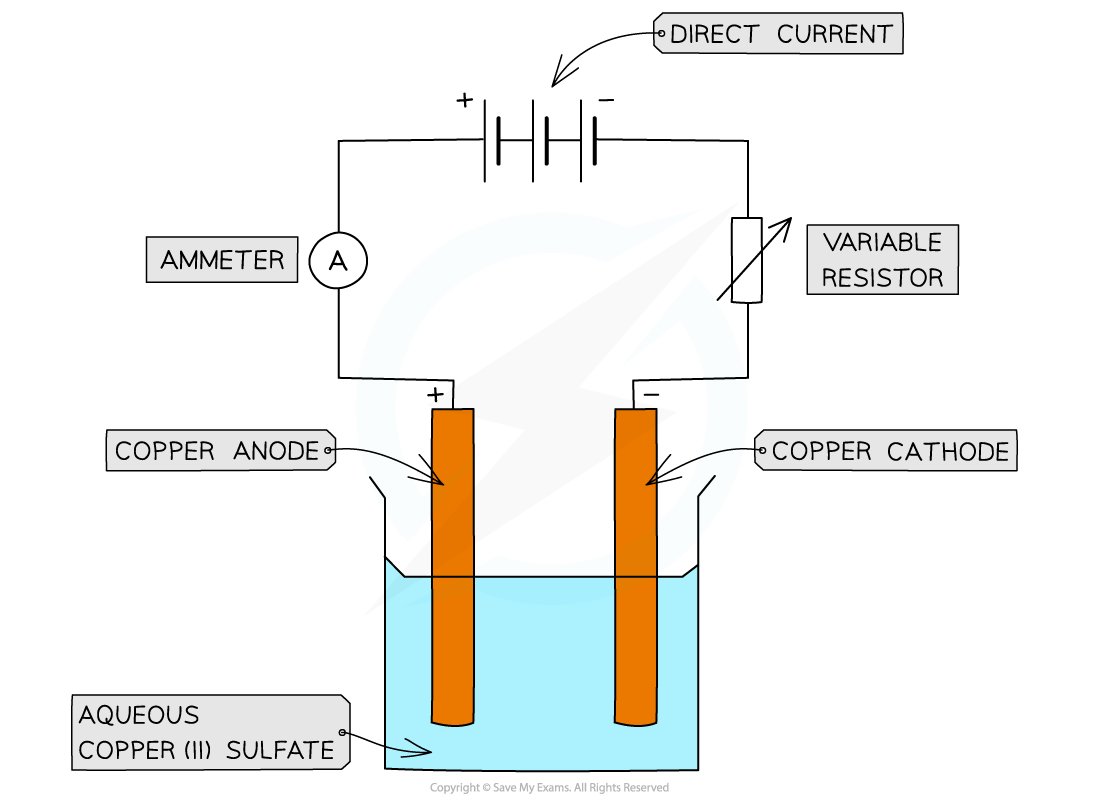
Cooper is used for both the anode and cathode
Method
Copper electrodes
Pour copper(II) sulfate solution into a beaker
Measure and record the mass of a piece of copper foil.
Attach it to the negative terminal of a DC supply, and dip the copper foil into the copper sulfate solution
Repeat step 2 and 3 with another piece of copper foil, but this time attach it to the positive terminal
Make sure the electrodes do not touch each other, then turn on the power supply
Adjust the power supply to achieve a constant current and leave for 20 minutes
Remove one of the electrodes and wash it with distilled water, then dip it into propanone
Lift the electrode out and allow all the liquid to evaporate. Do not wipe the electrodes clean.
Measure and record the mass of the electrode
Repeat with the other electrode making sure you can identify which electrode is which
Repeat the experiment with fresh electrodes and different currents
| Anode | Cathode |
Mass before (g) |
|
|
Mass after (g) |
|
|
Practical Tip
If you measure an electrode to see how much copper has been produced (or has been lost), ensure it is dry so you don't get a false reading
Analysis of results
Results
Results table
| Anode | Cathode |
Mass before (g) | 18.0 | 18.0 |
Mass after (g) | 18.4 | 17.6 |
Evaluation
Identify whether the mass of the electrodes has changed and by how much
The anode has increased in mass by 0.4 g
The cathode has decreased in mass by 0.4 g
Conclusion
The cathode increases in mass while the anode decreases
This occurs as copper atoms are oxidised at the anode and form ions while copper ions are reduced at the cathode, forming copper atoms
The gain in mass by the negative electrode is the same as the loss in mass by the positive electrode
Therefore the copper deposited on the negative electrode must be the same copper ions that are lost from the positive electrode
That implies that the concentration of the Cu2+ ions in the solution remains constant
Worked Example
The electrolysis of copper sulfate using graphite electrodes forms copper at the cathode.
i) State the observation at the anode
ii) Write the equation for the formation of copper at the cathode.
Answer
i) The observation at the anode is bubbling
ii) The equation for the formation of copper at the cathode is
Cu2+ + 2e– → Cu

Unlock more, it's free!
Did this page help you?