The Reactivity Series (OCR GCSE Chemistry A (Gateway)) : Revision Note
Reactions of Metals with Water
Metal atoms form positive ions by loss of electrons when they react with other substances
The tendency of a metal to lose electrons is a measure of how reactive the metal is
A more reactive metal loses electrons more easily than a less reactive one
Some metals react with water
Metals that react with cold water form a metal hydroxide and hydrogen gas:
metal + water → metal hydroxide + hydrogen
For example calcium:
Ca + 2H2O → Ca(OH)2 + H2
calcium + water → calcium hydroxide + hydrogen
Reactions of Metals with Acids
Most metals react with dilute acids such as HCl
When acids and metals react, the hydrogen atom in the acid is replaced by the metal atom to produce a salt and hydrogen gas:
metal + acid → metal salt + hydrogen
For example iron:
Fe + 2HCI → FeCl2 + H2
iron + hydrochloric acid → iron(II)chloride + hydrogen
In both these types of reactions (water and acids) the metals are becoming positive ions
The reactivity of the metals is related to their tendency to become an ion
The more reactive the metal the more easily it becomes an ion (by losing electrons)
The Reactions of Metals with Cold Water and Dilute Acids Summary Table

The reactions of metals and acids and metals and water allow them to be put into an order based on their reactivity, known as the reactivity series shown below
This can be done experimentally by observing the rate of reaction between them
The more reactive a metal is, the greater the rate of hydrogen production so the reaction will be more vigorous
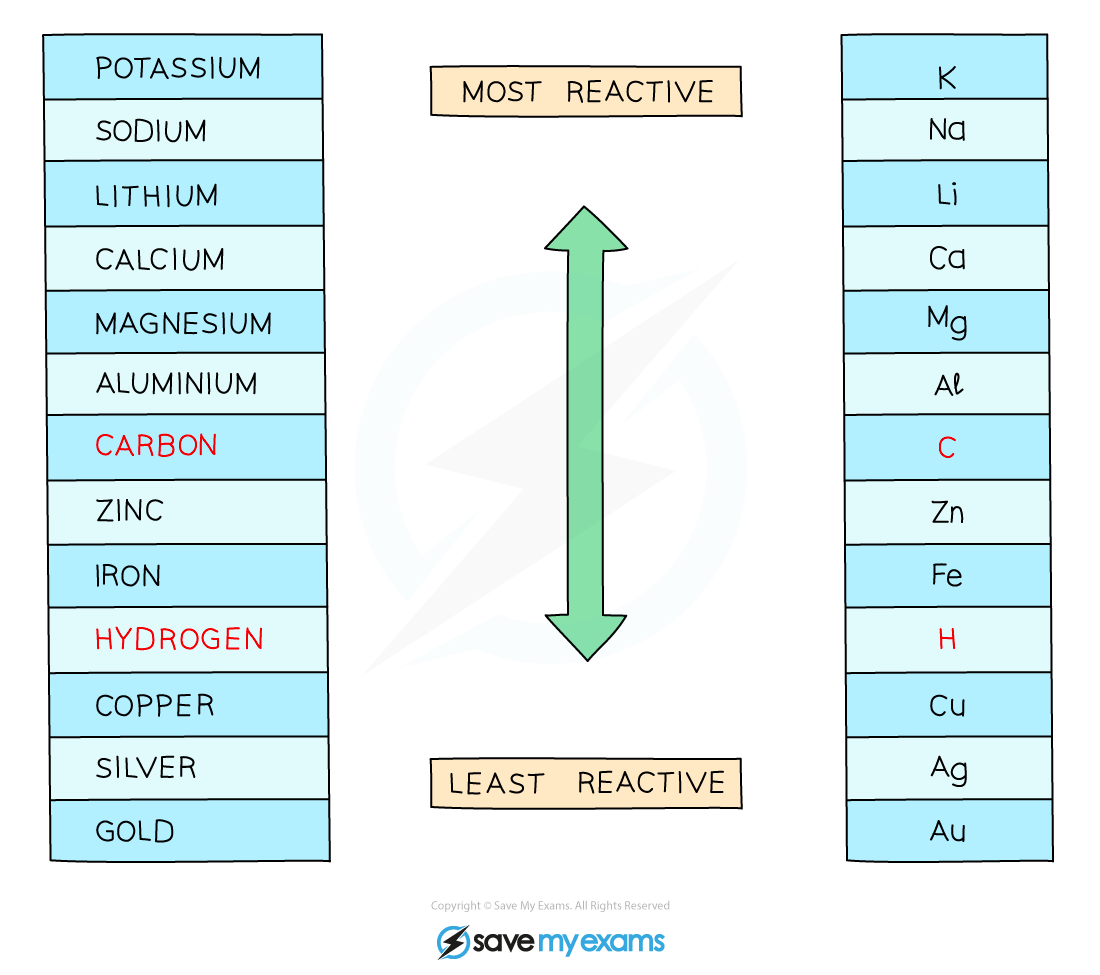
Diagram of the reactivity series of metals
There are several reactivity series mnemonics to help you remember the order of the metals
One that we like goes as follows: “Please send lions, cats, monkeys and cute zebras into hot countries signed Gordon"


You can learn the reactivity series with the help of a silly phrase
A reactivity series will usually contain the non-metal elements carbon and hydrogen
This is because these elements play different roles in our understanding the reactions of metals and our ability to predict how metals can be extracted from their ores
Reactions between metals and acids/water take place if the metal is able to displace the hydrogen in them
Carbon is a cheap reducing agent which can be used to remove oxygen from metal oxide ores
Placing carbon in the reactivity series allows us to see whether a metal oxide can be reduced or not by carbon
Metals below carbon can be extracted by heating the oxide with carbon
Metals higher than carbon have to be extracted by other methods, such as electrolysis
Displacement Reactions
The reactivity of metals decreases going down the reactivity series.
This means that a more reactive metal will displace a less reactive metal from its compounds
For example it is possible to reduce copper(II) oxide by heating it with zinc
The reducing agent in the reaction is zinc:
Zn + CuO → ZnO + Cu
zinc + copper oxide → zinc oxide + copper
Some other examples of displacement reactions can be found in the table below
Metal Solutions Displacement Table

Examiner Tips and Tricks
Displacement reactions occur when the solid metal is more reactive than the metal that is in the compound.

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
