Chromatography (OCR GCSE Chemistry A (Gateway)): Revision Note
Exam code: J248
Chromatography
Paper Chromatography
Chromatography is used to separate substances and provide information to help identify them
The components have different solubilities in a given solvent (e.g. different coloured inks that have been mixed to make black ink) and different adhesion to the supporting medium - usually paper
A pencil line is drawn on chromatography paper and spots of the sample are placed on it
Pencil is used for this as ink would run into the chromatogram along with the samples
The paper is then lowered into the solvent container, making sure that the pencil line sits above the level of the solvent so the samples don’t wash into the solvent container
The solvent travels up the paper by capillary action, taking some of the coloured substances with it
Different substances have different solubilities so will travel at different rates, causing the substances to spread apart
Those substances with higher solubility will travel further than the others
This is because they spend more time in the mobile phase and are thus carried further up the paper than the less soluble components

The pigments in ink can be analysed using paper chromatography
All chromatography techniques use two phases called the mobile phase and the stationary phase
In paper chromatography:
The mobile phase is the solvent in which the sample molecules can move, which in paper chromatography is liquid e.g. water or ethanol
The stationary phase in paper chromatography is the actual chromatography paper itself
Different dissolved substances have different affinities for the mobile and stationary phase which determines the speed they move through them
Thin- Layer Chromatography (TLC)
TLC works in a similar way to paper chromatography but has a different stationary phase
The stationary phase is a thin layer of an inert substance (e.g. silica) supported on a flat, unreactive surface
The mobile phase, like paper chromatography, is a solvent
Measuring Rf Values
These values are used to identify the components of mixtures
The Rf value of a particular compound is always the same but it is dependent, however, on the solvent used
If the solvent is changed then the value changes
Calculating the Rf value allows chemists to identify unknown substances because it can be compared with Rf values of known substances under the same conditions
These values are known as reference values
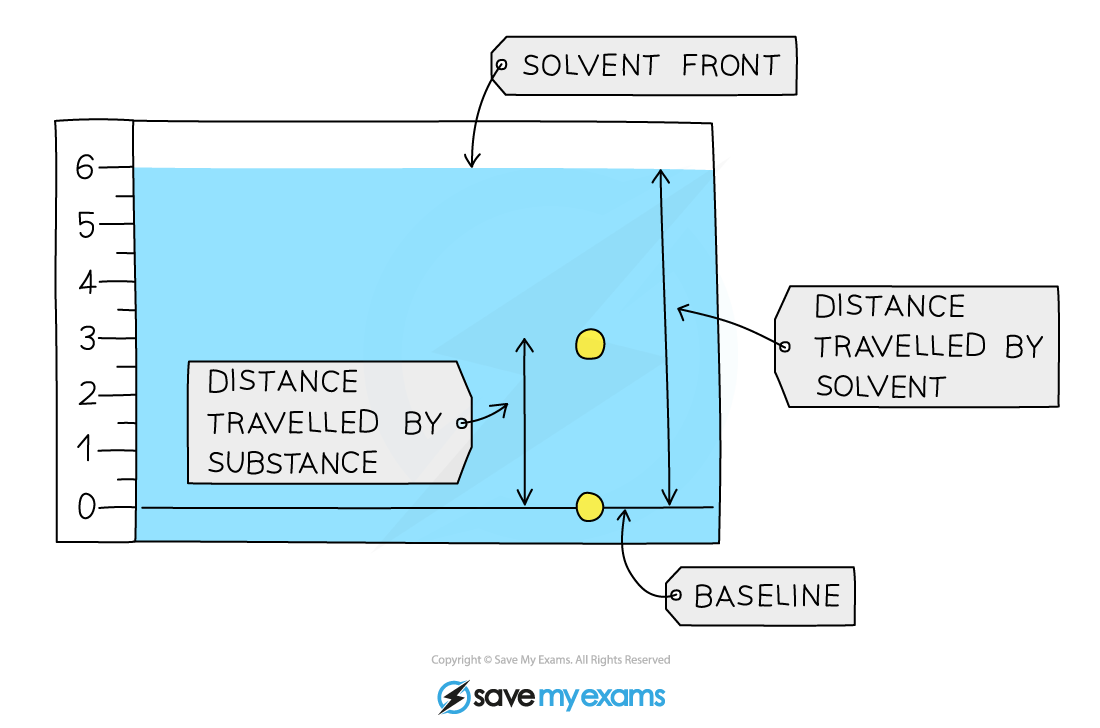
Calculation
Retention factor = distance moved by compound ÷ distance moved by solvent
The Rf value is a ratio and therefore has no units
Using Rf values to identify components of a mixture
Examiner Tips and Tricks
For the Rf calculations, both distances are measured from the baseline.
Types of Chromatography
Pure substances will produce only one spot on the chromatogram
If two or more substances are the same, they will produce identical chromatograms
If the substance is a mixture, it will separate on the paper to show all the different components as separate spots
An impure substance therefore will produce a chromatogram with more than one spot

Diagram showing the analysis of a mixture and pure substances using chromatography
Gas Chromatography
This is used to separates a mixture of gases
The mobile phase is an unreactive carrier gas e.g nitrogen
The stationary phase is a thin layer of an unreactive liquid e.g silica or aluminium powder
Gas chromatography occurs as follows:
The mixture sample is injected into the column
The mixture is carried by the carrier gas through the column
Different substances in the mixture will take different times to travel through the column (how long each one takes is known as the retention time and is due to their attraction to the stationary phase)
Substances with more attraction to the stationary phase will take longer to move through the column
As each component leaves the column, a peak is plotted again the travel time on a chromatogram generated by a computer

Interpreting Gas Chromatograms
A gas chromatogram tells you some key details about the mixture
the number of peaks- the number of compounds in the mixture
the height of the peak- how much of each compound is present (the higher the peak, the more compound there is)
the position of the peak- retention time of compound (how long it took to move through the column
Worked Example
Analysis of a compound by gas chromatography shows the presence of four components, A, B, C and D.

i) Which compound is present in the greatest quantity?
ii) Which compounds were present in equal amounts?
iii) Which compound had the strongest interaction with the stationary phase?
Answers:
i) D (the larger the relative size of the peak, the greater the quantity of that substance present)
ii) B and C (the peak sizes are equal)
iii) D (the larger the retention time, the greater the interaction of that component with the stationary phase)
Examiner Tips and Tricks
Make sure you can give the similarities and differences between types of chromatography

Unlock more, it's free!
Did this page help you?