Electrolysis of Aqueous Solutions (OCR GCSE Chemistry A (Gateway)): Revision Note
Exam code: J248
Electrolysis of Aqueous Solutions
Aqueous solutions will always have water (H2O)
In the electrolysis of aqueous solutions, the water molecules dissociate producing H+ and OH– ions:
H2O ⇌ H+ + OH–
These ions are also involved in the process and their chemistry must be considered
We now have an electrolyte that contains ions from the compound plus ions from the water
Which ions get discharged and at which electrode depends on the relative reactivity of the elements involved
Concentrated and dilute solutions of the same compound give different products
For anions, the more concentrated ion will tend to get discharged over a more dilute ion
Positive Electrode (anode)
Negatively charged OH– ions and non-metal ions are attracted to the positive electrode
If halide ions (Cl-, Br-, I-) and OH- are present then the halide ion is discharged at the anode, loses electrons and forms a halogen (chlorine, bromine or iodine)
If no halide ions are present, then OH- is discharged at the anode, loses electrons and forms oxygen
In both cases the other negative ion remains in solution
Negative Electrode (cathode)
Positively charged H+ and metal ions are attracted to the negative electrode but only one will gain electrons
Either hydrogen gas or the metal will be produced
If the metal is above hydrogen in the reactivity series, then hydrogen will be produced and bubbling will be seen at the cathode
This is because the more reactive ions will remain in solution, causing the least reactive ion to be discharged
Therefore at the cathode, hydrogen gas will be produced unless the positive ions from the ionic compound are less reactive than hydrogen, in which case the metal is produced
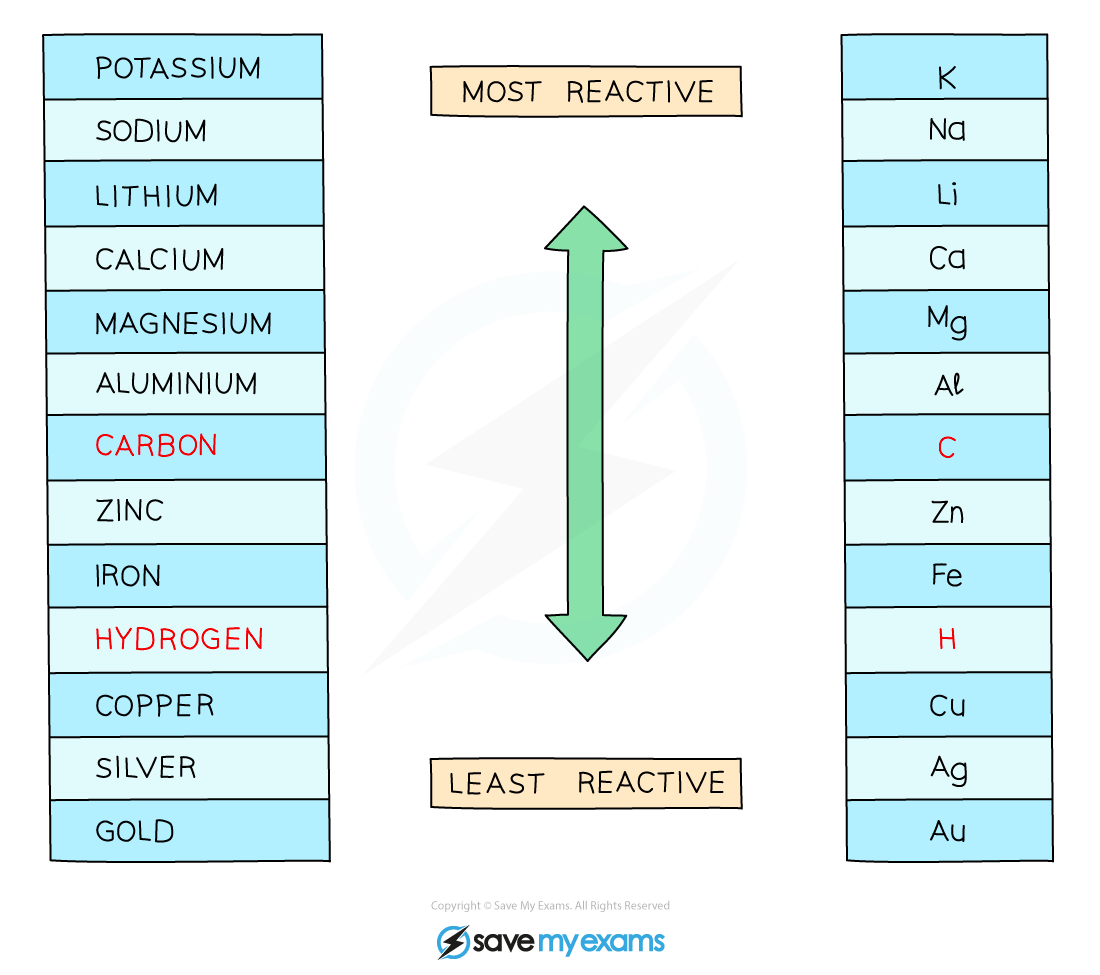
The reactivity series of metals including hydrogen and carbon
The electrode products are shown below for a series of common electrolytes
Common Electrolytes Table

Examiner Tips and Tricks
When answering questions on this topic, it helps if you first write down all of the ions present. Then compare their reactivity, work out which ones move to which electrode and deduce the products formed.
Inert & Non-Inert Electrodes
Inert Electrodes
Inert electrodes, such as graphite are usually used in electrolysis as they don't take part in the process, they just provide a surface for the reactions to happen on
The experimental process for the electrolysis of an aqueous solution using inert electrodes is:
Set up the apparatus as shown in the diagram
Add the aqueous solution to the beaker
Add two graphite rods as the electrodes and connect this to a power pack or battery
Turn on the power pack or battery and allow electrolysis to take place
Record the results in a suitable table (see below) and repeat for another solution, checking the electrodes in between runs to see if any metal has been deposited
The gases produced can be collected in the test tubes to be tested later

Diagram showing electrolysis using inert electrodes
Non-Inert Electrodes
Sometimes, non-inert or active electrodes are used which will take part in the electrolysis reactions
These are used for electroplating and purifing copper
Purifying copper is necessary as the copper obtained from its ore is not pure enough for use in, for example, electrical wires
Electroplating is a process used to coat metals in a thin layer of a different metal
Examples of this include:
coating copper or nickel jewellery with silver
coating taps with chromium
coating steel cutlery with silver
Purifying copper
The practical set up is similar to that of inert electrodes except both electrodes are made from copper
The anode is impure copper ore
The cathode is pure copper
The electrolyte is copper (II) sulfate solution
Once the power supply is turned up
At the anode Copper atoms lose electrons to become ions in the electrolyte
Cu ⟶ Cu2+ + 2e-
As a result the anode decreases in mass and impurities collect underneath
At the cathode the copper ions from the electrolyte gain electrons to form copper atoms
Cu2+ + 2e- ⟶ Cu
Copper collects on the cathode causing the mass to increase

Electroplating
The cathode is the object you want to coat
The anode is the metal you want to coat it with
The electrolyte is a solution containing ions of the coating metal
During the process:
At the anode metal atoms lose electrons to form ions in the electrolyte
At the cathode metal ions from the electrolyte gain electrons and are discharged on the surface of the metal that needs coating

Examiner Tips and Tricks
The key difference between inert and non-inert electrodes is that non-inert electrodes take part in the process, inert ones don't.
You only need to know the half equations for purifying copper if you are higher tier!

Unlock more, it's free!
Did this page help you?