This question is about ethene, the structure of which is shown in Figure 1.

Figure 1
Why is this molecule described as unsaturated?
Ethene can undergo a polymerisation reaction.
Complete the sentences using the words in the box.
high poly(ethene) polymers poly(ethane) ten monomers a few many |
During polymerization, ____________________ ethene molecules join together to form the large molecule, __________________.
These large molecules are called ___________________
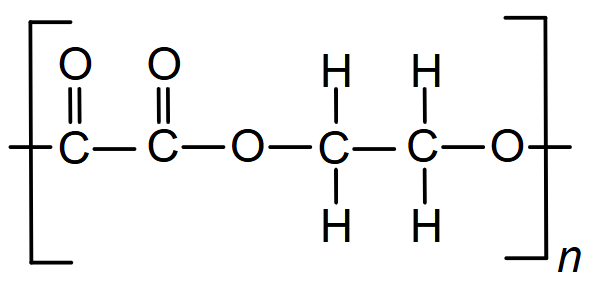
Complete the repeating unit of the polymer formed by ethene.

What type of polymer does ethene form?
Tick (✓) one box.
Condensation |
|
Addition |
|
Substitution |
|
Did this page help you?